Figure 1.
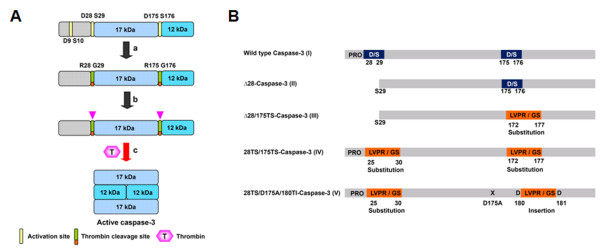
High-level expression of caspase-3 precursors in E. coli. A. Design of thrombin-activatable caspase-3 precursors resistant to autoactivation. B. Structure of designed proteins. I: wild-type caspase-3; II: caspase-3 devoid of the N-terminal 28 amino acids; III: Six amino acids (172–177) of construct II were substituted with LVPRGS (a sequence susceptible to thrombin activity); IV: Two sites (amino acid sequences 25–30 and 172–177) of wild-type caspase-3 were replaced with LVPRGS. V: Six amino acids (172–177) of wild-type caspase-3 were mutated to LVPRGS, and an additional LVPRGS motif inserted between Asp180 and Asp181. Asp175 was mutated to Ala.