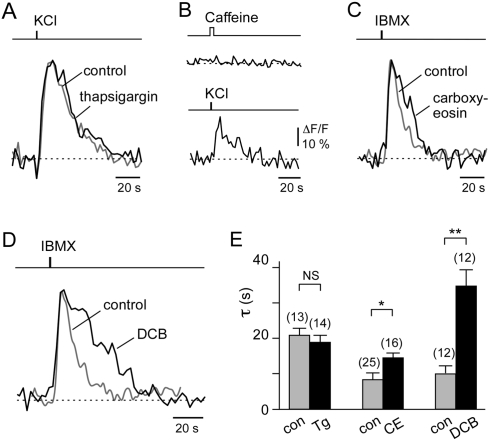
Figure 3. NCX is critical for reducing elevated intracellular Ca2+ levels in OSN knobs.
A, Comparison of the kinetic properties of the Ca2+ transients (normalized responses) in OSN knobs evoked by a 1-s pulse of KCl (80 mM) before (control) and after thapsigargin (200 nM) treatment. B, Ca2+ response (ΔF/F) of a single knob stimulated with a 5-s pulse of caffeine (10 mM) followed by a 1-s pulse of KCl (80 mM), confirming that thapsigargin (200 nM) pretreatment depleted Ca2+ from intracellular stores. C, Comparison of the kinetic properties of the Ca2+ transients (normalized responses) in OSN knobs evoked by a 1-s pulse of IBMX (100 µM) before (control) and after treatment with the PMCA inhibitor carboxyeosin (10 µM). D, Comparison of the kinetic properties of IBMX-induced Ca2+ transients (normalized responses) in OSN knobs before (control) and after treatment with the NCX inhibitor 3,4-dichlorobenzamil hydrochloride (DCB; 10 µM). E, Bar graphs showing collected decay time constants from control (gray) and treated (black) OSN knobs. For thapsigargin (Tg), control: τ = 21.1±1.9 s (n = 13, N = 4), Tg: τ = 19.0±1.9 s (n = 14, N = 4). For carboxyeosin (CE), control: τ = 8.5±1.9 s (n = 25, N = 8), CE: τ = 13.3±1.4 s (n = 16, N = 4). For 3,4-dichlorobenzamil hydrochloride (DCB), control: τ = 11.0±1.4 s (n = 12, N = 4), DCB: τ = 35.1±4.4 s (n = 12, N = 4). NS, not significant; *p<0.05; **p<0.0001. The Ca2+ transients in A,C, and D were rescaled to give the same peak amplitude.