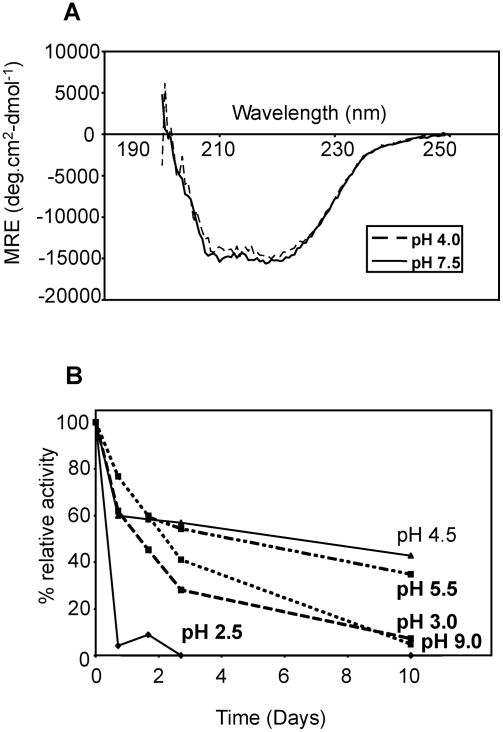
Figure 2. Stability of the zymogen proFheCL1Gly25 and mature FheCL1 at various pH values.
(A) Far-UV CD spectra of 5.3 µM FheproCL1Gly25 in 50 mM sodium acetate buffer, pH 4.0 and in 50 mM sodium phosphate buffer, pH 7.5. (B) Enzymatic stability of 6.0 µM mature FheCL1 at 37°C and in 0.1 M buffers over the pH range 2.5–9.0. Enzyme activity was monitored at various time-points by diluting aliquots of the reactions into 0.1 M sodium acetate buffer, pH 5.5, containing 3 mM DTT before addition of 5 µM Z-Phe-Arg-NMec.