Figure 5. Regulation of FheCL1 hydrolytic activity against haemoglobin by pH.
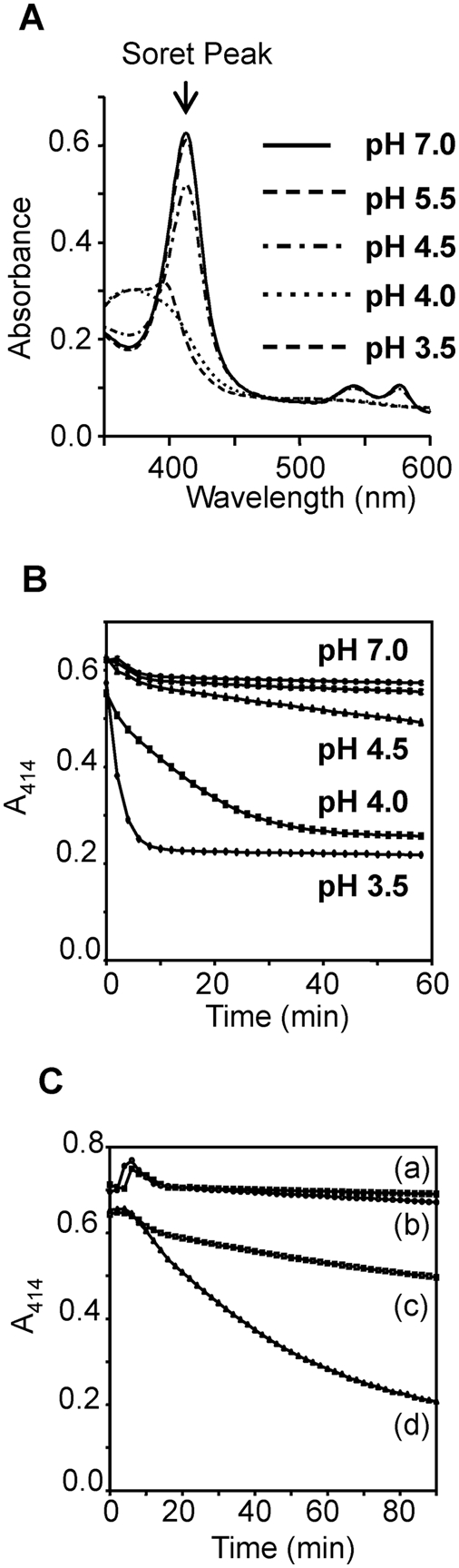
(A) Spectra of 5.0 µM Hb following 1 hr incubation in 0.1 M buffer at pH 3.5, pH 4.0, pH 5.5 and pH 7.0. Decreases in the Soret peak absorbance at 414 nm shows Hb denaturation with decreasing pH. (B) Progress of denaturation of 5.0 µM haemoglobin at several pH values over time as revealed by the decrease in absorbance at 414 nm. (C) Susceptibility of Hb to FheCL1 hydrolytic activity at pH 4.0 and pH 7.0 in the presence 1 mM GSH. (a) 5.0 µM Hb and 1 mM GSH at pH 7.0 (b) 5.0 µM Hb, 1 mM GSH and 1 µM FheCL1 at pH 7.0 (c) 5.0 µM Hb and 1 mM GSH at pH 4.5 (d) 5.0 µM Hb, 1 mM GSH and 1 µM FheCL1 at pH 4.5.
