Abstract
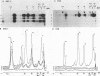
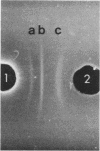
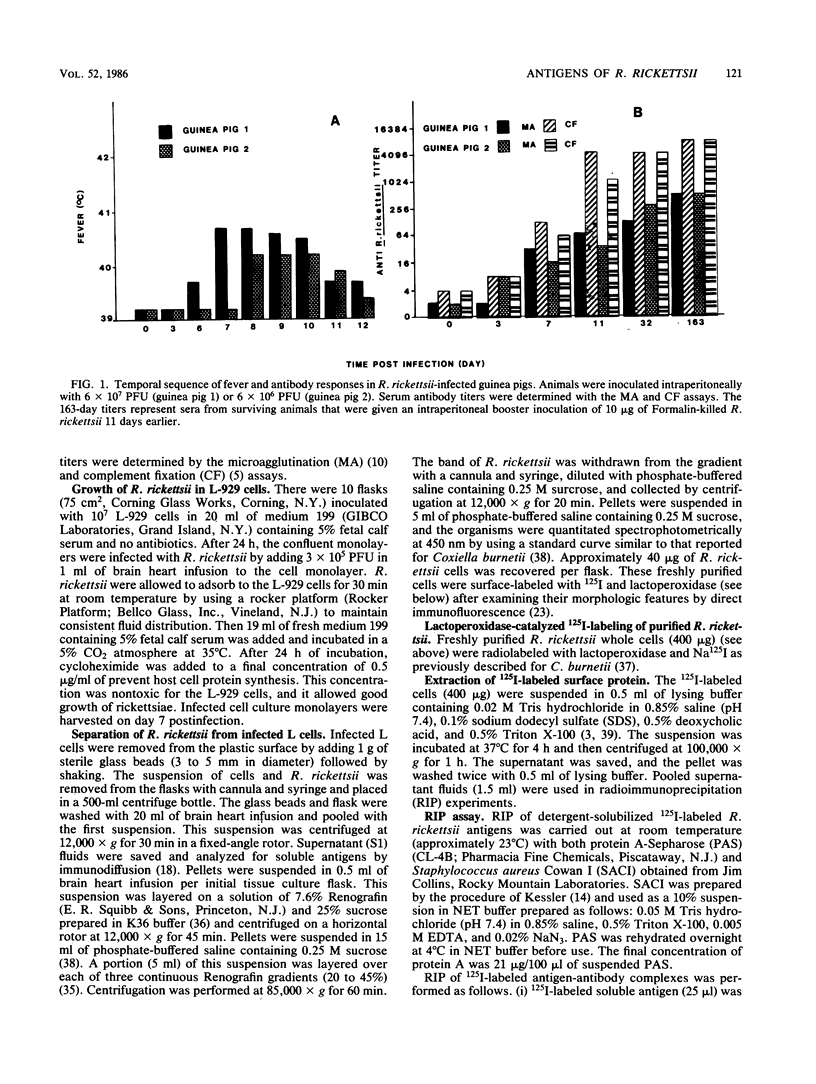
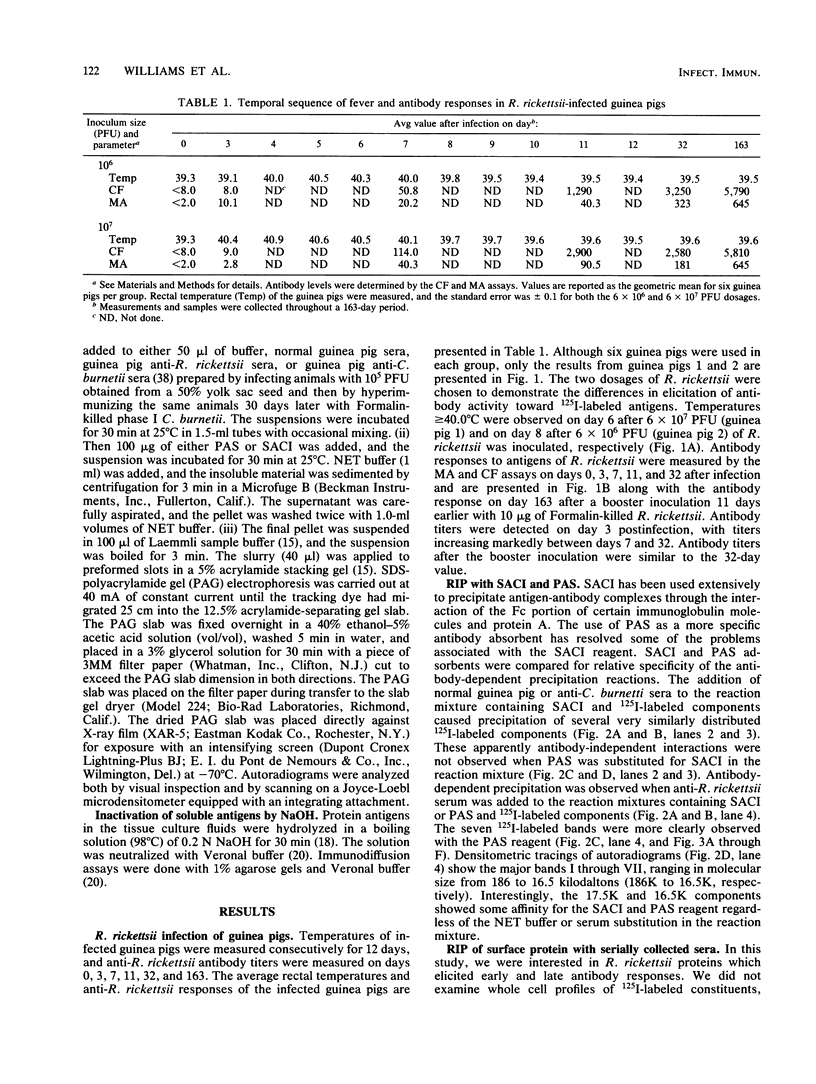
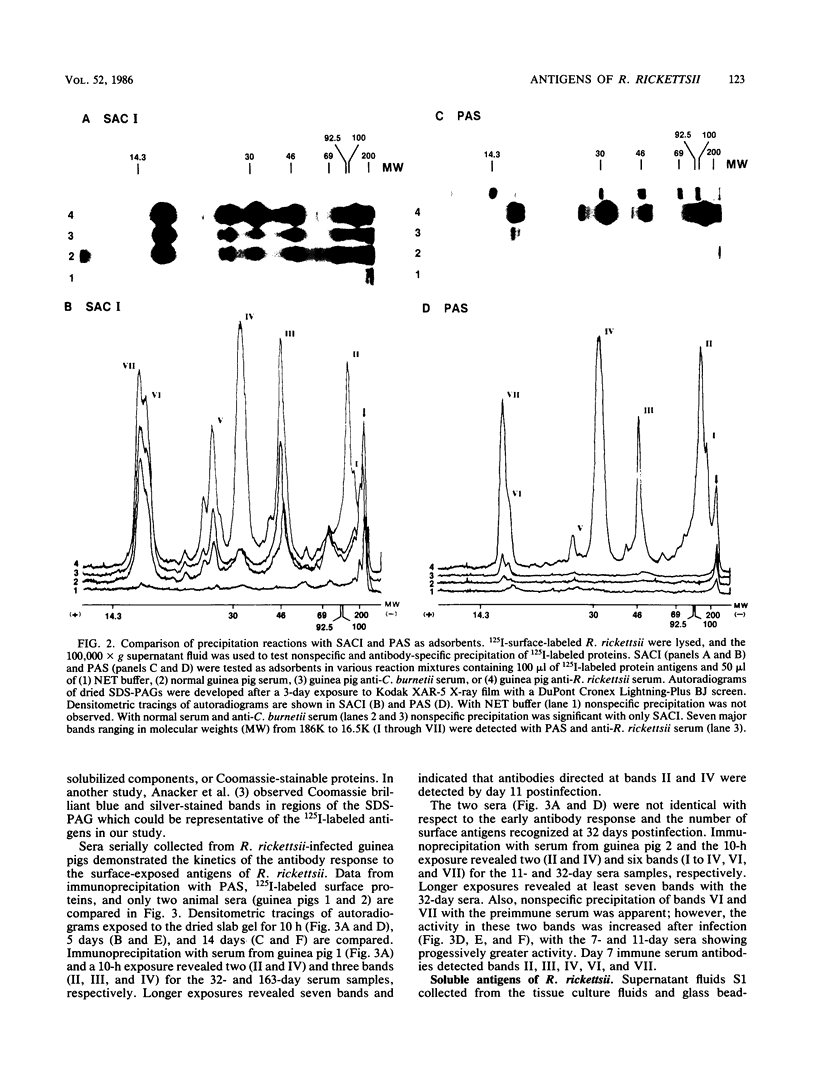
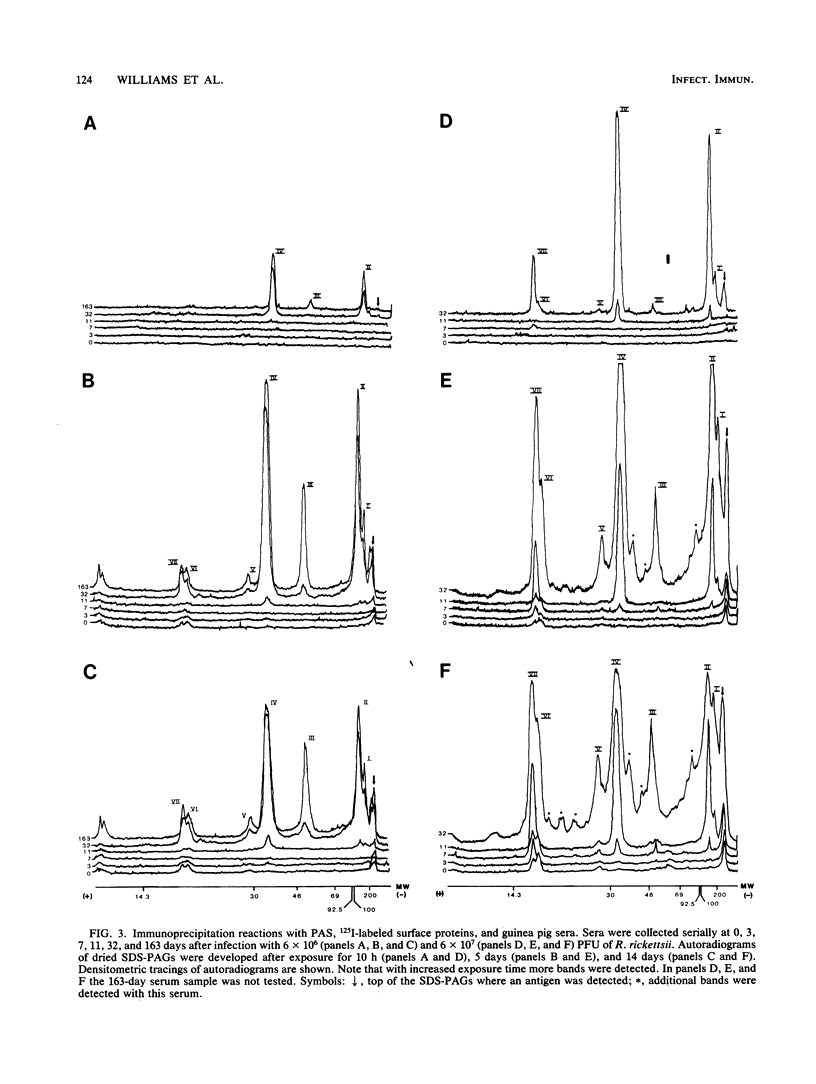
Rickettsia rickettsii, the etiologic agent of Rocky Mountain spotted fever, purified from infected L-929 cells by density gradient banding were extrinsically radioiodinated with lactoperoxidase. Immunodominant 125I-labeled antigens were identified by radioimmunoprecipitation of detergent-solubilized antigens with protein A-Sepharose and anti-R. rickettsii sera collected 0, 3, 7, 11, 32, and 163 days after infection of guinea pigs. The average fever greater than or equal to 40 degrees C was detected by days 3 and 4 after infection with 6 X 10(7) and 6 X 10(6) PFU, respectively. By microagglutination and complement fixation assays, anti-R. rickettsii antibodies were detected as early as day 3 after infection, with titers increasing markedly between days 7 and 163. Convalescent sera, collected on day 163, from infected guinea pigs were used to identify seven 125I-labeled antigens with apparent molecular sizes of 186,000 (I), 145,000 (II), 49,000 (III), 32,000 (IV), 27,500 (V), 17,500 (VI), and 16,500 (VII) daltons. Differences in antibody reactivity and specificity against the seven antigens were demonstrated with serially obtained sera. Sera from a guinea pig infected with 6 X 10(7) PFU exhibited antibody-antigen interactions with all seven 125I-labeled antigens by day 7, whereas the same antibody activity required 32 days for an animal infected with 6 X 10(6) PFU. Prominent antibody activities toward proteins II and IV were demonstrated both early and late after infection. The fluids obtained from infected L-929 cells contained three soluble antigens which were detected with the 11-, 32-, and 163-day sera by an immunodiffusion assay. The soluble and 125I-labeled antigens of R. rickettsii identified in this study may be important candidates for vaccines against Rocky Mountain spotted fever.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Casper E., Todd W. J., Mann R. E., Johnston M. R., Nauck C. J. Biological properties of rabbit antibodies to a surface antigen of Rickettsia rickettsii. Infect Immun. 1983 Apr;40(1):292–298. doi: 10.1128/iai.40.1.292-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Williams J. C., List R. H., Mann R. E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher M. S., Oster C. N., Harber P. I., Kenyon R. H., Pedersen C. E., Jr Initial clinical evaluation of a new Rocky Mountain spotted fever vaccine of tissue culture origin. J Infect Dis. 1978 Aug;138(2):217–221. doi: 10.1093/infdis/138.2.217. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Wisseman C. L., Jr, Woodward T. E., Fiset P., Dumler J. S., McNamee W., Black R. E., Rooney J., Hughes T. P., Levine M. M. Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J Infect Dis. 1983 Nov;148(5):922–930. doi: 10.1093/infdis/148.5.922. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Dawkins A. T., Heiner G. G., Fabrikant I. B., Wisseman C. L., Jr, Woodward T. E. Rocky Mountain spotted fever: a comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii. J Infect Dis. 1973 Sep;128(3):340–344. doi: 10.1093/infdis/128.3.340. [DOI] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Fishbein D. B., Kaplan J. E., Bernard K. W., Winkler W. G. Surveillance of Rocky Mountain spotted fever in the United States, 1981-1983. J Infect Dis. 1984 Oct;150(4):609–611. doi: 10.1093/infdis/150.4.609. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Pedersen C. E., Jr Preparation of Rocky Mountain spotted fever vaccine suitable for human immunization. J Clin Microbiol. 1975 Jun;1(6):500–503. doi: 10.1128/jcm.1.6.500-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. G., Jr, Fiset P. Mechanisms of immunity in typhus infection: adoptive transfer of immunity to Rickettsia mooseri. Infect Immun. 1979 May;24(2):387–393. doi: 10.1128/iai.24.2.387-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Osterman J. V., Eisemann C. S. Rickettsial indirect hemagglutination test: isolation of erythrocyte-sensitizing substance. J Clin Microbiol. 1978 Aug;8(2):189–196. doi: 10.1128/jcm.8.2.189-196.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. J. Production of gamma interferon in mice immune to Rickettsia tsutsugamushi. Infect Immun. 1984 Jan;43(1):59–65. doi: 10.1128/iai.43.1.59-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Walters V. D. Comparative electrophoresis of spotted fever group rickettsial proteins. Life Sci. 1978 Feb;22(7):583–587. doi: 10.1016/0024-3205(78)90337-5. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- STOENNER H. G., LACKMAN D. B., BELL E. J. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr Comparative ultrastructural study on the cell envelopes of Rickettsia prowazekii, Rickettsia rickettsii, and Rickettsia tsutsugamushi. Infect Immun. 1978 Sep;21(3):1020–1023. doi: 10.1128/iai.21.3.1020-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of the properties of antirickettsial activity and interferon in mouse lymphokines. Infect Immun. 1983 Oct;42(1):27–32. doi: 10.1128/iai.42.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos T., Palmer E. L., Obijeski J. F., Martin M. L. Origin and structure of the group-specific, complement-fixing antigen of Rickettsia rickettsii. Appl Microbiol. 1974 Sep;28(3):481–488. doi: 10.1128/am.28.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Henderson F. W. Effect of immunosuppression on Rickettsia rickettsii infection in guinea pigs. Infect Immun. 1978 Apr;20(1):221–227. doi: 10.1128/iai.20.1.221-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]