Abstract
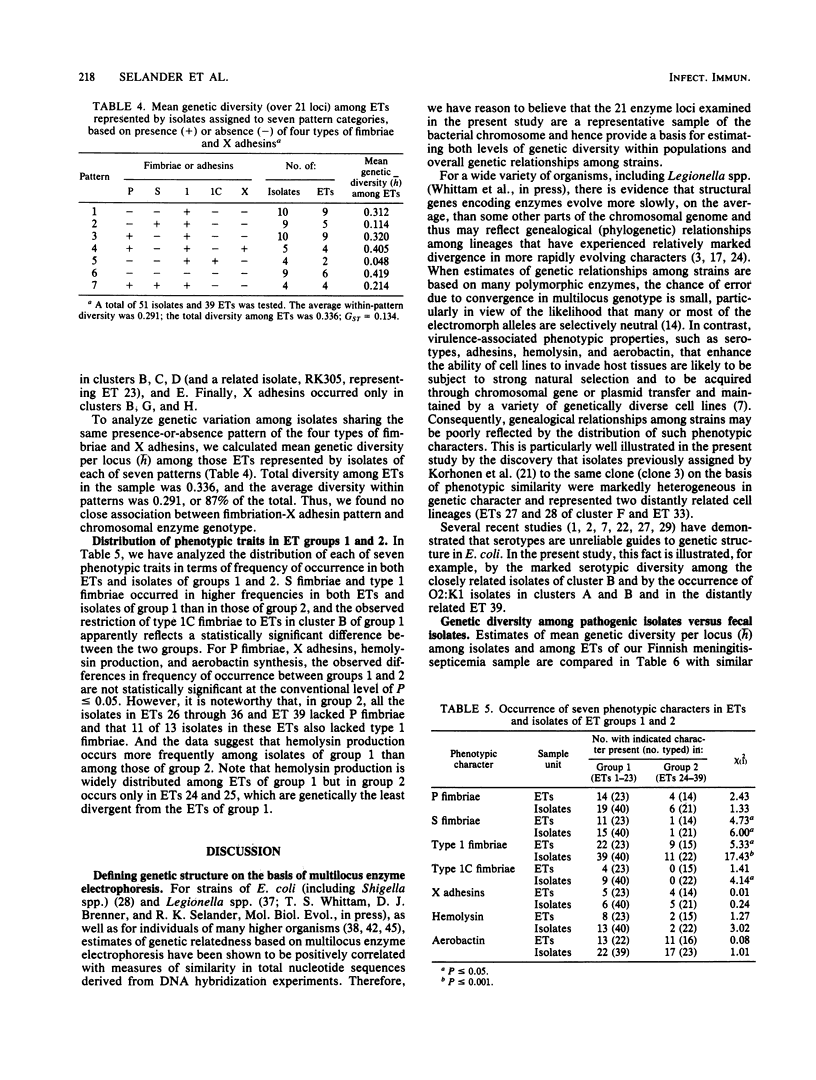
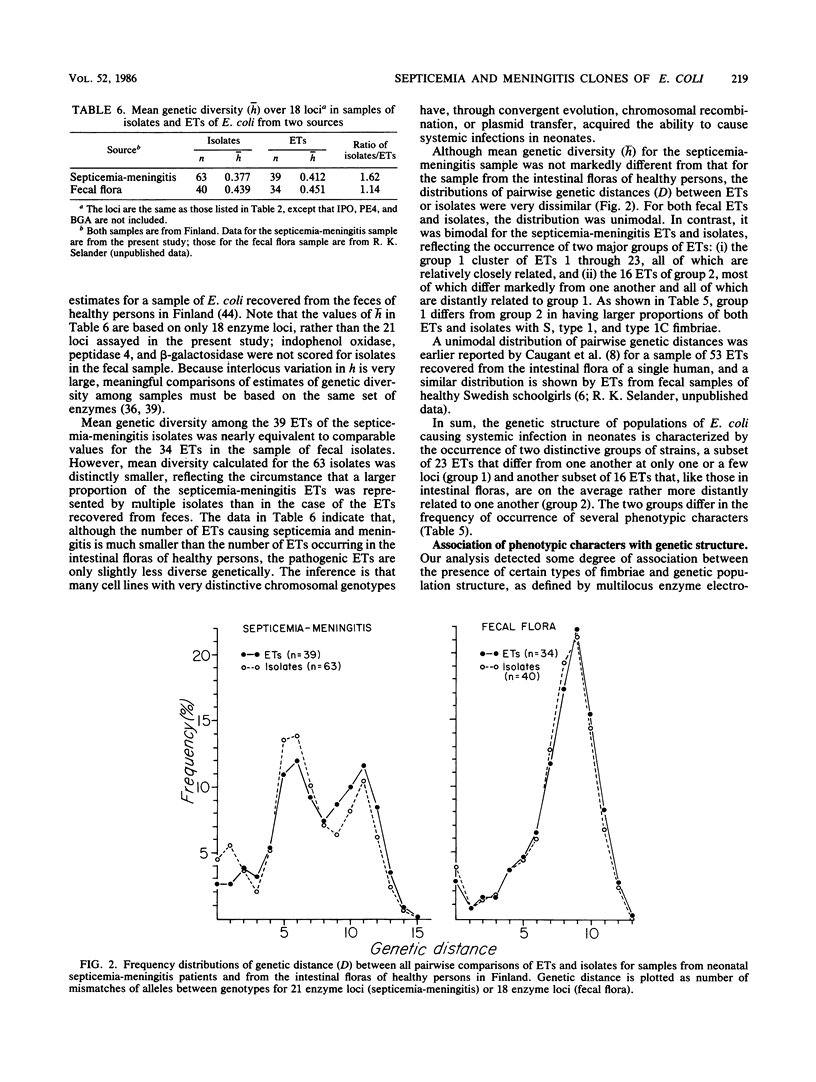
Genetic diversity and relationships among 63 isolates of Escherichia coli from infants in Finland with septicemia or meningitis were assessed by analyzing electrophoretic variation in 21 enzymes encoded by chromosomal genes. Thirty-nine multilocus genotypes (electrophoretic types) were distinguished, 23 of which formed a closely related, distinctive subset (group 1) of five or six clones represented by 40 (63%) of the isolates. The remaining isolates represented a second subset of 16 electrophoretic types (group 2) that were, on the average, rather more distantly related to one another. Although the number of electrophoretic types causing neonatal systemic disease is smaller than that occurring in healthy intestinal floras, the pathogenic electrophoretic types are only slightly less diverse genetically. Isolates of group 1 were characterized by relatively high incidences of hemolysin production and S, type 1, type 1C, and P fimbriae. However, because phenotypic characters, considered individually or in combination, did not adequately reflect the overall genetic relationships of isolates, it is recommended that the genetic structure of populations be defined on the basis of multilocus chromosomal genotypes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Heuzenroeder M., Kusecek B., Ochman H., Caugant D., Selander R. K., Väisanen-Rhen V., Korhonen T. K., Stuart S., Orskov F. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect Immun. 1986 Jan;51(1):268–276. doi: 10.1128/iai.51.1.268-276.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Lidin-Janson G., Whittam T. S., Svanborg Edén C., Selander R. K. Genetic diversity and relationships among strains of Escherichia coli in the intestine and those causing urinary tract infections. Prog Allergy. 1983;33:203–227. doi: 10.1159/000318331. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Orskov I., Orskov F., Svanborg Eden C., Selander R. K. Genetic diversity in relation to serotype in Escherichia coli. Infect Immun. 1985 Aug;49(2):407–413. doi: 10.1128/iai.49.2.407-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Hull S., Hull R., Pruckler J. Construction and comparison of recombinant plasmids encoding type 1 fimbriae of members of the family Enterobacteriaceae. Infect Immun. 1985 May;48(2):275–279. doi: 10.1128/iai.48.2.275-279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal K. K., Warner P. J., Williams P. H. An inducible outer membrane protein involved in aerobactin-mediated iron transport by co1V strains of Escherichia coli. FEBS Lett. 1982 Apr 5;140(1):27–30. doi: 10.1016/0014-5793(82)80513-9. [DOI] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E. The population genetics of Escherichia coli. Annu Rev Genet. 1984;18:31–68. doi: 10.1146/annurev.ge.18.120184.000335. [DOI] [PubMed] [Google Scholar]
- Hull S. I., Hull R. A., Minshew B. H., Falkow S. Genetics of hemolysin of Escherichia coli. J Bacteriol. 1982 Aug;151(2):1006–1012. doi: 10.1128/jb.151.2.1006-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull S., Clegg S., Sanborg Eden C., Hull R. Multiple forms of genes in pyelonephritogenic Escherichia coli encoding adhesins binding globoseries glycolipid receptors. Infect Immun. 1985 Jan;47(1):80–83. doi: 10.1128/iai.47.1.80-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. A., Hall T. J., Britten R. J. Evolutionary distances in Hawaiian Drosophila measured by DNA reassociation. J Mol Evol. 1981;17(6):361–367. doi: 10.1007/BF01734358. [DOI] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Morelli G., Heuzenroeder M., Kamke M., Achtman M. Conservation of plasmids among Escherichia coli K1 isolates of diverse origins. Infect Immun. 1984 Dec;46(3):649–657. doi: 10.1128/iai.46.3.649-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet. 1977 Oct;41(2):225–233. doi: 10.1111/j.1469-1809.1977.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pere A., Leinonen M., Väisänen-Rhen V., Rhen M., Korhonen T. K. Occurrence of type-1C fimbriae on Escherichia coli strains isolated from human extraintestinal infections. J Gen Microbiol. 1985 Jul;131(7):1705–1711. doi: 10.1099/00221287-131-7-1705. [DOI] [PubMed] [Google Scholar]
- Rhen M. Characterization of DNA fragments encoding fimbriae of the uropathogenic Escherichia coli strain KS71. J Gen Microbiol. 1985 Mar;131(3):571–580. doi: 10.1099/00221287-131-3-571. [DOI] [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Templeton A. R. The phylogeny of the hominoid primates: a statistical analysis of the DNA-DNA hybridization data. Mol Biol Evol. 1985 Sep;2(5):420–433. doi: 10.1093/oxfordjournals.molbev.a040363. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]


