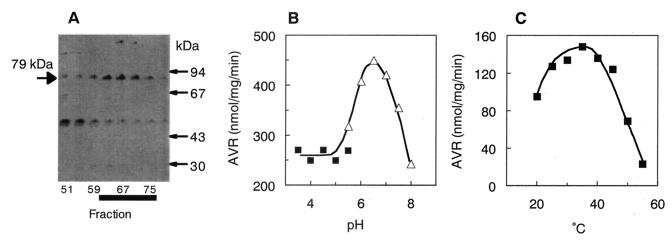
FIG. 3.
Characterization of purified OAVN cyclase. (A) SDS-PAGE of partially purified OAVN cyclase. The proteins in the active fractions eluted from the Resource PHE column were electrophoresed in an SDS-polyacrylamide gel and stained with Coomassie brilliant blue R-250. OAVN cyclase appeared as a 79-kDa band. Fractions that had high OAVN cyclase activity are marked with a thick black line. (B) AVR formation from OAVN was measured under various pH conditions. (▪), sodium acetate buffer; (▵), sodium phosphate buffer. (C) AVR formation from OAVN was measured at various temperatures.