FIG. 5.
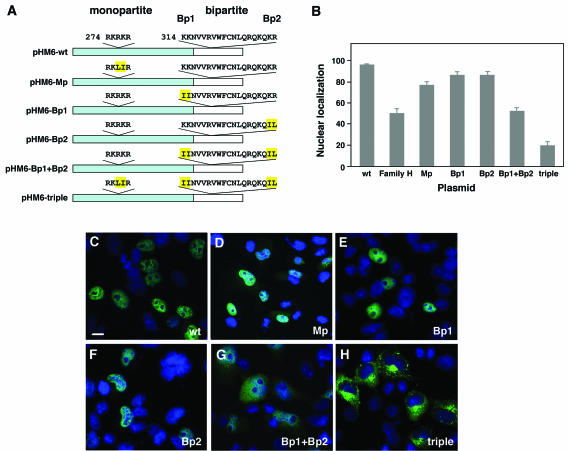
POU4F3 contains two functional NLS. (A) Schematic representation of the constructs containing wild-type POU4F3 and NLS mutations. The portion missing in the family H mutant protein is shown in white. The monopartite (Mp) and bipartite (Bp1 and Bp2) NLS sequences are indicated. Amino acids that were mutated are highlighted in yellow. (B) The graph displays the relative fluorescence intensities in the nucleus versus the whole cell after immunostaining with anti-HA antibody (mean ± the SD). (C to H) In transfected COS-7 cells, the localization of different mutant NLS constructs was analyzed. Nuclei were stained with DAPI (blue), and HA-fused proteins (green) were visualized by confocal microscopy. Immunolocalization of pHM6-Mp was both nuclear and cytoplasmic (D), and that of pHM6-Bp1 (E) and pHM6-Bp2 (F) was nuclear, similar to the cellular localization of pHM6-wild-type POU4F3 (wt) (C). Mutating both parts of the bipartite NLS (pHM6-Bp1+Bp2) led to partial cytoplasmic localization (G). Mutating both the monopartite and bipartite NLS (pHM6-Mp+Bp1+Bp2 or triple) resulted in complete cytoplasmic accumulation of the protein (H), indicating that both NLS are required for nuclear localization of POU4F3. Scale bar, 10 μm.