Abstract
Rhodium catalyzed hydrogenation of 1,3-enynes 1a-8a and 1,3-diynes 9a-13a at ambient temperature and pressure in the presence of ethyl (N-tert-butanesulfinyl)iminoacetate and ethyl (N-2,4,6-triisopropylbenzenesulfinyl)iminoacetates, respectively, results in reductive coupling to afford unsaturated α-amino acid esters 1b-13b in good to excellent yields with exceptional levels of regio- and stereocontrol. Further hydrogenation of the diene containing α-amino acid esters 1b-8b using Wilkinson’s catalyst at ambient temperature and pressure results in regioselective reduction to afford the β,γ-unsaturated α-amino acid esters 1c-8c in good to excellent yields. Exhaustive hydrogenation of the unsaturated side chains of the Boc- and Fmoc-protected derivatives of enyne and diyne coupling products 14b-16b occurs in excellent yield using Crabtree’s catalyst at ambient temperature and pressure to afford the α-amino acid esters 14d-16d, which possess saturated side chains. Finally, cross-metathesis of the Boc-protected reductive coupling product 14b with cis-1,4-diacetoxy-2-butene proceeds readily to afford the allylic acetate 14e. Isotopic labeling studies that involve reductive coupling of enyne 1a and diyne 9a under an atmosphere of elemental deuterium corroborate a catalytic mechanism in which oxidative coupling of the alkyne and imine residues is followed by hydrogenolytic cleavage of the resulting metallacycle. A stereochemical model accounting for the observed sense of asymmetric induction is provided. These studies represent the first use of imines as electrophilic partners in hydrogen-mediated reductive carbon-carbon bond formation.
Introduction
Reductive methods for catalytic C-C bond formation have emerged as the subject of intensive investigation.1-7 The direct catalytic reductive coupling of alkenes,1 alkynes,2 allenes,3 enones,4,6a-d 1,3-dienes,2,6e 1,3-enynes5,6f and 1,3-diynes6g to carbonyl partners have been reported. Inspired by the prospect of developing completely atom economical variants of such transformations, hydrogen-mediated C-C bond formation has become the focus of research in our lab.6,7 Hydrogen-mediated reductive couplings to carbonyl partners using conjugated enones,6a-d dienes,6e enynes6f and diynes6g have been achieved, as well as hydrogen-mediated reductive cyclizations of 1,6-diynes and 1,6-enynes.6h,i These studies are among the first examples of hydrogen-mediated C-C bond formation that proceed in absence of carbon mononoxide.8,9
To further broaden this emergent class of reductive couplings, the use of imines as electrophilic partners in hydrogen-mediated C-C bond formation was explored. Whereas three component couplings of alkynes, organoboranes and imines are reported,10 simple hydrometallative variants are unknown. Here, we disclose that hydrogenation of 1,3-enynes or 1,3-diynes in the presence of ethyl (N-sulfinyl)iminoacetates11,12 enables highly regio- and stereoselective reductive coupling to afford unnatural diene- and enyne containing α-amino acid esters.13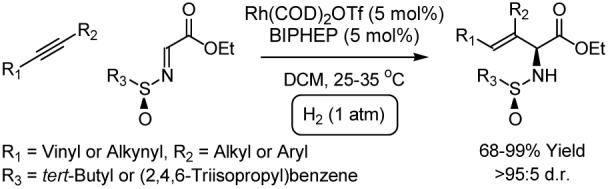
Results and Discussion
Reductive Coupling of Conjugated Alkynes and N-Sulfinyl Iminoacetates
Initial efforts focused on the hydrogen-mediated coupling of 1,3-enyne 1a (200 mol%) to assorted (N-sulfinyl)iminoacetates (100 mol%) at ambient pressure and temperature in dichloromethane (0.1 M). The rhodium precatalyst was generated in situ from Rh(COD)2OTf (5 mol%) and BIPHEP (5 mol%). It was found that hydrogenation of 1a in the presence of ethyl (N-para-toluenesulfinyl)iminoacetate (100 mol%) provides the desired reductive coupling product in 95% yield, but with poor control of diastereoselectivity. A further decrease in stereoselection is observed in conjunction with the use of the corresponding mesityl derivative. However, hydrogenation of 1,3-enyne 1a in presence of ethyl (N-tert-butanesulfinyl)iminoacetate furnishes reductive coupling product 1b in 55% yield as a single regio- and stereoisomer. When the reaction is conducted at higher concentration (0.3 M), the yield is increased to 92% without loss of selectivity. Finally, at slightly elevated temperature (35° C), 1b is obtained in 99% yield as a single diastereomer (Scheme 1).
Scheme 1.
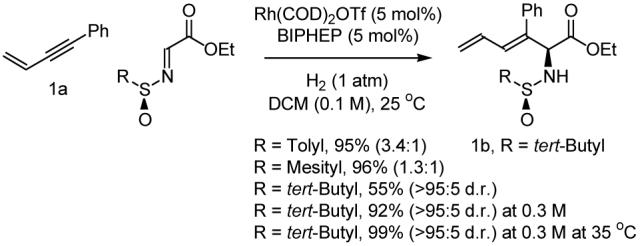
Optimization of the hydrogen-mediated reductive coupling of 1,3-enyne 1a to various ethyl (N-sulfinyl)iminoacetates.
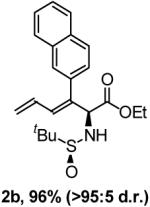
The use of ethyl (N-tert-butanesulfinyl)iminoacetate under these conditions proved to be general across a range of structurally diverse enynes 1a-8a. In all cases examined, >95:5 regio- and diastereoselectivity is observed (Table 1). The chemoselectivity of the hydrogen-mediated coupling is underscored by the fact that over-reduction of the diene-containing products 1b-8b is not observed. The regio- and stereochemical assignment of reductive coupling products 1b-8b is described in the supporting information, and is based upon crystallographic analysis of a derivative of 4b.
Table 1.
Hydrogen-mediated reductive coupling of 1,3-enynes 1a-8a and ethyl (N-tert-Butanesulfinyl)iminoacetates and regioselective hydrogenation of coupling products 1b-8b to provide β,γ-unsaturated α-amino acid esters 1c-8ca
| Entry | Substrate | Coupling Product | mono-Reduction Product |
|---|---|---|---|
| 1 |  |
 |
 |
| 2 |  |
 |
 |
| 3 | 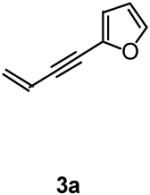 |
 |
 |
| 4 |  |
 |
 |
| 5 |  |
 |
 |
| 6 |  |
 |
 |
| 7 |  |
 |
 |
The reductive coupling of 1a-8a was performed at ambient temperature, in accordance with the reaction conditions cited in Scheme 1 at 0.3 M and at 35 °C under otherwise identical conditions. The reductive couplings were complete in less than 8 hours. The partial hydrogenation of 1b-8b to provide β,γ-unsaturated amino acid esters 1c-8c was performed at ambient pressure and temperature using Wilkinson’s catalyst in toluene solvent. See experimental section for detailed reaction conditions.
The hydrogen-mediated reductive coupling of 1,3-diynes to (N-sulfinyl)iminoacetates was explored next. For the purpose of optimization, the rhodium catalyzed hydrogenation of 1-phenyl-1,3-pentadiyne 9a (200 mol%) in the presence of assorted (N-sulfinyl)iminoacetates (100 mol%) at ambient pressure and temperature in dichloromethane (0.1 M) was studied. Interestingly, under conditions established for the reductive coupling of enynes 1a-8a, coupling of diyne 9a to ethyl (N-tert-butanesulfinyl)iminoacetate is not observed, presumably due to the diminished electrophilicity of the alkyl substituted sufinyl imine. In contrast, hydrogenation of diyne 9a in the presence of (N-para-toluenesulfinyl)iminoacetate delivers a 94% yield of the desired coupling product 9b as a 1.2:1 mixture of diastereomers. Remarkably, 9b appears as a single regioisomer, as coupling occurs exclusively at the aromatic terminus of the conjugated diyne 9a. Upon use of the corresponding mesityl derivative, coupling product 9b is obtained 96% yield with >95:5 regio- and diastereocontrol. Comparable results were obtained in conjunction with the use of ethyl N-(2,4,6-triisopropyl)benzenesulfinyl iminoacetate, which was adopted as the standard coupling partner due to its enhanced chromatographic stability and, hence, ease of purification (Scheme 2).
Scheme 2.

Optimization of the hydrogen-mediated reductive coupling of 1,3-diyne 9a to various ethyl (N-sulfinyl)iminoacetates.
The coupling of 1,3-diynes 9a-13a to ethyl N-(2,4,6-triisopropyl)benzenesulfinyl iminoacetate under the aforementioned conditions provides the enyne-containing α-amino acid esters 9b-13b as single regio- and stereoisomers in good to excellent yield. The regioisomeric products 9c-12c are not observed. The stereochemical assignment of 9b-13b is made in analogy to that established via single crystal x-ray diffraction analysis for a derivative of 4b, as described in the supporting information.
Elaboration of Reductive Coupling Products
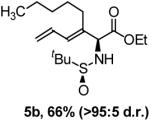
In a preliminary effort to explore further manipulation of the coupling products, compound 1a was exposed to Wilkinson’s catalyst at ambient pressure and temperature.5,14 In the event, regioselective hydrogenation of the terminal alkene occurs cleanly to afford the β,γ-unsaturated α-amino esters 1c in 74% yield, without over-reduction to the fully saturated derivative 1d. These regioselective hydrogenation conditions were applied to all enyne coupling products 1b-8b. The β,γ-unsaturated α-amino esters 1c-8c were obtained in 70-93% yield (Table 1). Exhaustive hydrogenation of the diene side chain using Crabtree’s catalyst was explored next.15 Here, the N-tert-butanesulfinyl residue must be exchanged for a carbamate protecting group, as in 14b and 15b. Hydrogenation of 14b using Crabtree’s catalyst at ambient pressure and temperature provides the fully saturated Boc-protected amino acid ester 14d in 99% as a 2:1 mixture of diastereomers. Under identical conditions, the saturated Fmoc-protected amino acid ester 14d is obtained in 95% as a 2.4:1 mixture of diastereomers. Conversion of 9b to the corresponding Boc-derivative 16b followed by exhaustive hydrogenation of the enyne side chain using Crabtree’s catalyst provides the saturated amino acid ester 16d in excellent yield. The relative stereochemistry of 14d-16d was not assigned (Scheme 3).
Scheme 3.
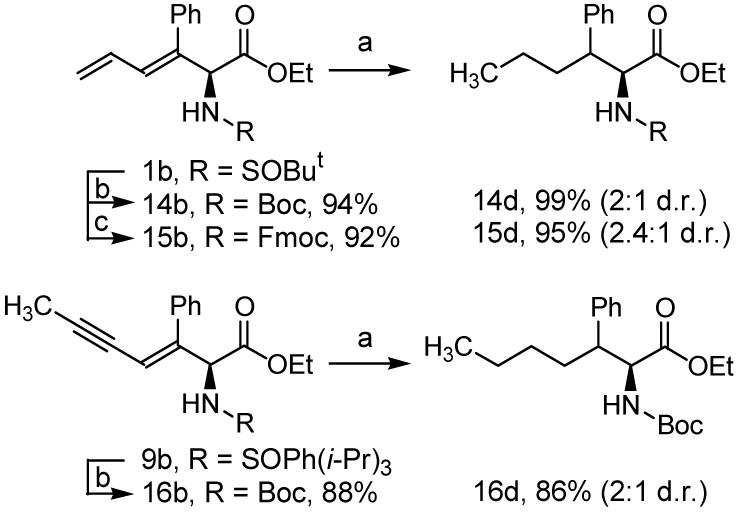
Exhaustive hydrogenation of the diene and enyne side chains of coupling product 1b, 14b and 15b and exhaustive hydrogenation of the enyne side chain of 16b.
Whereas enynes possessing vinylic substitution couple readily to glyoxal partners under hydrogenation conditions,6f the present enyne-iminoacetate couplings do not tolerate vinylic substitution. Hence, functionalization of the diene terminus via cross-metathesis was explored. Gratifyingly, exposure of the Boc-protected reductive coupling product 14b to cis-1,4-diacetoxy-2-butene in the presence of substoichiometric quantities of the second generation Grubb’s catalyst provides the allylic acetate 14e in 80% yield as a 10:1 mixture of alkene stereoisomers (Scheme 4).16
Scheme 4.

Cross-metathesis of 14b to afford allylic acetate 14e.
Catalytic Mechanism and Model for Stereoinduction
Under an atmosphere of elemental deuterium, rhodium catalyzed reductive coupling of 1a and ethyl (N-tert-butanesulfinyl)iminoacetate provides mono-deuterio-1b. Similarly, the reductive coupling of 9a to ethyl N-(2,4,6-triisopropyl)benzenesulfinyl iminoacetate under a deuterium atmosphere provides mono-deuterio-9b. These results are consistent with a catalytic mechanism involving alkyne-imine oxidative coupling followed by hydrogenolytic cleavage of the resulting metallacycle. An analogous mechanism for related hydrogen-mediated carbocyclizations catalyzed by rhodium has been inferred on the basis of more detailed mechanistic studies.6i The hydrogenolytic cleavage of the metallacyclic intermediate likely involves hydrogen activation via σ-bond metathesis. An increasing body of evidence supports participation of organorhodium(III) intermediates in σ-bond metathesis pathways,17 including reactions with hydrogen.17c Factors dictating the regiochemistry of C-C bond formation remain uncertain. However, previously disclosed competition experiments6f suggest back-bonding from low valent rhodium to the bound alkyne, which enables generation of a nucleophilic metallacyclopropene, may play a decisive role.18 Nucleophilic activation of alkynes through complexation by low valent early transition metals is well established.19 For low valent late transition metals, this pattern of reactivity may represent a driving force that assists the oxidative coupling of alkynes to C=O π-bonds, as in the Ni(0) catalyzed reductive coupling of alkynes and aldehydes.2,3 The modest nucleophilic character of a Rh(I)-alkyne complex may account for the requirement of highly activated electrophilic partners such as iminoacetates and, as previously reported, glyoxals (Scheme 5).6f,6g
Scheme 5.

Proposed catalytic mechanism as corroborated by deuterium labeling.
Given the observed sense of stereoinduction, two steereochemical models A and B are proposed. In each case, the iminoacetate is bound to rhodium(I) in a 5-membered chelate. This mode of coordination has been established for late transition metal complexes of α-iminoketones and α-iminoesters.20 Upon oxidative coupling, the alkyne is delivered to the less encumbered π-face of the imine, as dictated by the anticipated conformational preference of the N-sulfinyl imine.21 The resulting metallacycle C possesses an unoccupied coordinate site L, which should facilitate subsequent hydrogen activation (Scheme 6).
Scheme 6.
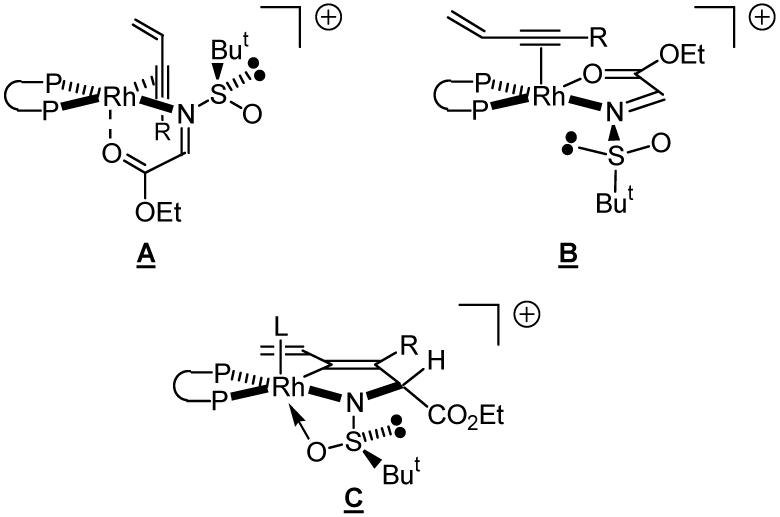
Proposed models for asymmetric induction.
Summary
In this account, the reductive coupling of conjugated alkynes and iminoacetates is achieved through catalytic hydrogenation, representing the first use of imines as electrophilic partners in simple hydrometallative reductive coupling. Good to excellent yields and exceptional levels of regiocontrol are observed. Moreover, the high levels of relative stereocontrol with respect to the N-sulfinyl moiety allow the absolute stereochemical course of the reductive coupling to be directed.
Initial studies on the elaboration of the reductive coupling products reveal that the unsaturated side chain of the diene-containing products 1b-8b may be partially hydrogenated to afford the corresponding β,γ-unsaturated α-amino acid esters 1c-8c. Exhaustive hydrogenation of both diene and enyne containing reductive couplings is possible, as demonstrated by the hydrogenation of compounds 14b-16b to furnish 14d-16d, which possess completely saturated side chains. Finally, as demonstrated by the conversion of 14b-14e, cross-metathesis of the diene containing α-amino acid esters occurs in good yield.
The outcome of isotopic labeling studies, which involve the reductive coupling of enyne 1a and diyne 9a to iminoacetates under an atmosphere of elemental deuterium, are consistent with a catalytic mechanism involving oxidative coupling of the alkyne and imine residues followed by hydrogenolytic cleavage of the resulting metallacyclic intermediate. The requirement of π-unsaturated reactants in the form of activated imines and conjugated alkynes suggest alkyne coordination by low valent rhodium to form a weakly nucleophilic metallocyclopropene. For low valent late transition metals, this pattern of reactivity may represent a driving force that assists the oxidative coupling of alkynes to C=O π-bonds.
Experimental Section
General
Anhydrous solvents were transferred by an oven-dried syringe. Flasks were flame-dried and cooled under a stream of nitrogen. Dichloromethane (DCM) was distilled from calcium hydride. Analytical thin-layer chromatography (TLC) was carried out using 0.2-mm commercial silica gel plates (DC-Fertigplatten Kieselgel 60 F254). Preparative column chromatography employing silica gel was performed according to the method of Still. 1H-NMR spectra were recorded with a Varian Gemini (400 MHz or 300 MHz) spectrometer. Chemical shifts are reported in delta (δ) units, parts per million (ppm) downfield from trimethylsilane. Coupling constants are reported in Hertz (Hz). Carbon-13 nuclear magnetic resonance (13C-NMR) spectra were recorded with a Varian Gemini 400 (100 MHz) spectrometer. Chemical shifts are reported in delta (δ) units, ppm relative to the center of the triplet at 77.0 ppm for deuteriochloroform. 13C NMR spectra were routinely run with broadband decoupling.
Representative Procedure for the Reductive Coupling of 1,3-Enynes and N-(tert-Butanesulfinyl)iminoacetate
To a solution of N-(tert-Butanesulfinyl)iminoacetate (41 mg, 0.2 mmol, 100 mol%) and 3-buten-1-ynyl-benzene 1a (50.9 mg, 0.4 mmol, 200 mol%) in DCM (0.67 mL, 0.3M) at 35 °C was added Rh(COD)2OTf (4.7 mg, 0.01 mmol, 5 mol%) and BIPHEP (5.3 mg, 0.01 mmol, 5 mol%). The system was purged with argon gas followed by hydrogen gas. The reaction was allowed to stir at 35 °C under 1 atm of hydrogen until complete consumption of substrate, at which point the reaction mixture was evaporated onto silica gel and the product purified by silica gel chromatography.
2-(2-methyl-propane-2-sulfinylamino)-3-phenyl-hexa - 3,5-dienoic acid ethyl ester (1b)
1H NMR (400 MHz, CDCl3): 7.34-7.26 (m, 3H), 7.19-7.16 (m, 2H), 6.42 (d, J = 10.8 Hz, 1H), 6.30 (dt, J = 16.8, 10.4 Hz, 1H), 5.15 (dd, J = 10.0, 1.6 Hz, 1H), 5.14 (dd, J = 10.0, 1.6 Hz, 1H), 4.80 (d, J = 4.0 Hz, 1H), 4.34 (d, J = 4.0, 1H), 4.17 (q, J = 7.2 Hz, 2H), 1.19 (s, 9H), 1.18 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.0, 137.9, 137.0, 133.3, 132.6, 129.3, 128.0, 127.6, 120.1, 62.8, 62.0, 55.9, 22.5, 13.9. HRMS: Calcd. for C18H26N1O3S1 [M+1] 336.1633, found 336.1632. FTIR (neat): 3285, 3072, 2980, 1738, 1602, 1494, 1474, 1444, 1366, 1296, 1256, 1219, 1184, 1076, 914, 851, 776 cm-1.
2-(2-methyl-propane-2-sulfinylamino)-3-naphthalen-2-yl-hexa-3,5-dienoic acid ethyl ester (2b)
1H NMR (400 MHz, CDCl3): 7.84-7.78 (m, 3H), 7.66 (s, 1H), 7.51-7.46 (m, 2H), 7.30 (dd, J = 8.4, 1.6 Hz, 1H), 6.51 (d, J = 11.2 Hz, 2H), 6.35 (t, J = 10.4 Hz, 1H), 6.51 (d, J = 11.2 Hz, 1H), 6.30 (dt, J = 17.2, 10.4 Hz, 1H), 5.40 (dd, J = 16.8, 1.4 Hz, 1H), 5.16 (dd, J = 10.2, 1.4 Hz, 1H), 4.90 (d, J = 4.4 Hz, 1H), 4.41 (d, J = 4.0, 1H), 4.20-4.15 (m, 2H), 1.19 (s, 9H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.0, 137.9, 134.5, 133.3, 133.0, 132.9, 132.6, 128.5, 127.9, 127.6, 127.2, 126.2, 126.2, 120.3, 62.8, 62.1, 56.0, 22.5, 14.0. HRMS: Calcd. for C22H28N1O3S1 [M+1] 386.1790, found 386.1786. FTIR (neat): 3283, 2979, 1733, 1473, 1366, 1258, 1219, 1184, 1075, 914, 859, 824, 751 cm-1.
2-(2-methyl-propane-2-sulfinylamino)-3-furan-2-yl-hexa-3,5-dienoic acid ethyl ester (3b)
1H NMR (400 MHz, CDCl3): 7.42 (d, J = 1.2, 1H), 7.12 (dt, J = 17.6, 10.7, 1H), 6.42 (d, J = 3.2 Hz, 1H), 6.40-6.38 (m, 1H), 6.30 (d, J = 11.2 Hz, 1H), 5.46 (d, J = 8.4 Hz, 1H), 5.36 (dd, J = 10.0, 1.2 Hz, 1H), 4.80 (d, J = 3.2 Hz, 1H), 4.44 (d, J = 2.8 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 1.19 (t, J = 7.2 Hz, 3H), 1.15 (s, 9H). 13C NMR (100 MHz, CDCl3): 171.0, 150.8, 142.2, 133.4, 132.4, 125.9, 111.0, 110.8, 62.2, 61.4, 55.7, 22.4, 13.9. HRMS: Calcd. for C16H24N1O4S1 [M+1] 326.1426, found 326.1426. FTIR (neat): 3287, 2980, 1737, 1467, 1366, 1260, 1224, 1114, 1072, 1021 cm-1.
3-(1H-Indol-2-yl)-2-(2-methyl-propane-2-sulfinyl amino)-hexa-3,5-dienoic acid ethyl ester (4b)
1H NMR (400 MHz, CDCl3): 8.90 (s, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.16 (td, J = 7.6, 1.1 Hz, 1H), 7.07 (t, J = 7.4 Hz, 1H), 6.90 (dt, J = 17.2, 10.6 Hz, 1H), 6.55 (d, J = 1.6 Hz, 1H), 6.48 (d, J = 10.8, 1H), 5.50 (d, J = 16.0 Hz, 1H), 5.33 (d, J = 10.0 Hz, 1H), 4.85 (d, J = 3.6, 1H), 4.46 (d, J = 3.2 Hz, 1H), 4.15 (q, J = 7.2 Hz, 2H), 1.20 (s, 9H), 1.11 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): 172.2, 136.0, 134.8, 133.4, 133.2, 127.9, 127.8, 122.5, 122.0, 120.5, 120.0, 110.9, 105.2, 62.4, 61.8, 56.0, 22.5, 13.7. HRMS: Calcd. for C20H27N2O3S1 [M+1] 375.1742, found 375.1747. FTIR (neat): 3274, 2978, 1731, 1455, 1404, 1366, 1224, 1056, 913, 850, 795, 783 cm-1. MP: 50 - 52 °C.
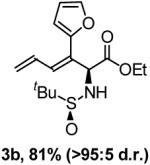
3-Allylidene-2-(2-methyl-propane-2-sulfinylamino)-octanoic acid ethyl ester (5b)
1H NMR (400 MHz, CDCl3): 6.58 (dt, J = 16.8, 10.4 Hz, 1H), 6.11 (d, J = 11.2 Hz, 1H), 5.27 (dd, J = 16.8, 1.6 Hz, 1H), 5.19 (dd, J = 9.8, 1.4 Hz, 1H), 4.45 (d, J = 4.0, 1H), 4.32 (d, J = 4.0 Hz, 1H), 4.27 -4.17 (m, 2H), 2.19 (t, J = 8.0, 2H), 1.44 - 1.36 (m, 2H), 1.29-1.26 (m, 7H), 1.26 (s, 9H), 0.88 (t, J = 2.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.6, 138.0, 132.1, 130.6, 118.9, 62.0, 62.0, 55.8, 31.9, 28.6, 28.6, 22.6, 22.4, 14.0, 14.0. HRMS: Calcd. for C17H32N1O3S1 [M+1] 330.2103, found 330.2090. FTIR (neat): 3452, 2956, 2869, 1734, 1465, 1365, 1255, 1182, 1078, 908, 851 cm-1.
3-(tert-Butyl-dimethyl-silanyloxymethyl)-2-(2-methyl-propane-2-sulfinylamino)-hexa-3,5-dienoic acid ethyl ester (6b)
1H NMR (400 MHz, CDCl3): 6.62 (dt, J = 16.8, 10.5 Hz, 1H), 6.13 (d, J = 10.8 Hz, 1H), 5.32 (dd, J = 15.2, 1.4 Hz, 1H), 5.24 (dd, J = 10.0, 1.6 Hz, 1H), 4.59 (d, J = 4.4 Hz, 1H), 4.44-4.38 (m, 2H), 4.32 -4.23 (m, 2H), 4.17-4.41 (m, 1H), 1.27 (t, J = 1.2, 3H), 1.25 (s, 9H), 0.09 (s, 9H), 0.062 (s, 6H). 13C NMR (100 MHz, CDCl3): 171.4, 136.4, 131.2, 131.1, 120.3, 61.8, 60.1, 58.6, 55.8, 25.8, 22.6, 18.3, 14.0, -5.52. HRMS: Calcd. for C19H38N1O4Si1S1 [M+1] 404.2291, found 404.2299. FTIR (neat): 3418, 2956, 2925, 2857, 1735, 1472, 1365, 1255, 1212, 1077, 838, 778, 736 cm-1.
3-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-2-(2-methyl-propane-2-sulfinylamino)-hexa-3,5-dienoic acid ethyl (7b)
1H NMR (400 MHz, CDCl3): 6.55 (dt, J = 16.8, 10.6 Hz, 1H), 6.12 (d, J = 10.8, 2H), 5.24 (dd, J = 16.8, 1.6 Hz, 1H), 5.17 (dd, J = 10.2, 1.6 Hz, 1H), 4.45 (d, J = 4.8 Hz, 1H), 4.32 (d, J = 4.4, 1H), 4.25-4.11 (m, 2H), 3.63-3.53 (m, 2H), 2.48-2.34 (m, 2H), 1.24 (t, J = 6.4 Hz, 3H), 1.21 (s, 9H), 0.84 (s, 9H), 0.018 (s, 6H). 13C NMR (100 MHz, CDCl3): 171.4, 134.1, 132.3, 132.1, 119.5, 62.4, 62.1, 62.0, 55.9, 32.2, 25.9, 22.6, 18.2, 14.0, -5.37. HRMS: Calcd. for C20H40N1O4Si1S1 [M+1] 418.2447, found 418.2447. FTIR (neat): 3447, 3283, 2956, 2929, 2857, 1734, 1646, 1472, 1388, 1365, 1255, 1181, 1086, 989, 912, 836, 776 cm-1.
3-(tert-Butoxycarbonylamino-methyl)-2-(2-methyl-propane-2-sulfinylamino)-hexa-3,5-dienoic acid ethyl ester (8b)
1H NMR (400 MHz, CDCl3): 6.67 (dt, J = 16.4, 10.4 Hz, 1H), 6.26 (d, J = 11.2 Hz, 1H), 5.39 (d, J = 16.4 Hz, 1H), 5.32 (d, J = 10.0 Hz, 1H), 4.58 (s, 1H), 4.53 (d, J = 3.6 Hz, 1H), 4.45 (s, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.94 (d, J =5.2 Hz, 1H), 1.43 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 1.27 (s, 9H). 13C NMR (100 MHz, CDCl3): 170.9, 155.3, 134.5, 132.9, 131.1, 121.4, 79.4, 62.2, 61.2, 55.8, 37.3, 28.2, 22.5, 14.0. HRMS: Calcd. for C18H33N2O5S1 [M+1] 389.2110, found 389.2108. FTIR (neat): 3384, 2978, 1734, 1707, 1507, 1457, 1391, 1366, 1251, 1170, 1064, 917, 734 cm-1.
deuterio-2-(2-methyl-propane-2-sulfinylamino)-3-phenyl-hexa-3,5-dienoic acid ethyl ester (deuterio-1b)
1H NMR (400 MHz, CDCl3): 7.34-7.25 (m, 3H), 7.18-7.15 (m, 2H), 6.29 (dd, J = 16.8, 10.0 Hz, 1H), 5.35 (dd, J = 17.0, 1.8 Hz, 1H), 5.13 (dd, J = 10.2, 1.8 Hz, 1H), 4.79 (d, J = 4.8 Hz, 1H), 4.34 (d, J = 4.4, 1H), 4.16 (q, J = 7.2 Hz, 2H), 1.17 (s, 9H), 1.17 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.0, 137.8, 137.0, 133.2, 129.3, 128.0, 127.6, 120.1, 62.8, 62.1, 55.9, 22.5, 13.9. HRMS: Calcd. for C18H25D1N1O3S1 [M+1] 337.1696, found 337.1692. FTIR (neat): 3285, 3056, 2980, 2870, 1732, 1601, 1493, 1473, 1444, 1412, 1391, 1366, 1253, 1220, 1074, 911, 852, 735, 703, cm-1.
Representative Procedure for the Regioselective Hydrogenation of Dienes
To a solution of diene 1b (33.5 mg, 0.1 mmol, 100 mol%) in toluene (1 mL, 0.1M) at ambient temperature was added RhCl(PPh3)3 (9.3 mg, 0.01 mmol, 10 mol%). The system was purged with argon gas followed by hydrogen gas. The reaction was allowed to stir at ambient temperature under 1 atm of hydrogen until complete consumption of substrate, at which point the reaction mixture was evaporated onto silica gel and the product purified by silica gel chromatography.
2-(2-Methyl-propane-2-sulfinylamino)-3-phenyl-hex-3-enoic acid ethyl ester (1c)
1H NMR (400 MHz, CDCl3): 7.32-7.23 (m, 3H), 7.13-7.10(m, 2H), 5.83 (t, J = 7.4 Hz, 1H), 4.72 (d, J = 4.4 Hz, 2H), 4.23 (d, J = 4.0 Hz, 1H), 4.20-4.12 (m, 2H), 1.99 (qt, J = 7.4 Hz, 2H), 1.19 (t, J = 7.0 Hz, 3H), 1.19 (s, 9H), 0.95 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.4, 137.3, 136.0, 135.6, 129.1, 127.9, 127.2, 63.1, 61.8, 55.8, 22.5, 22.3, 14.0, 13.9. HRMS: Calcd. for C18H28N1O3S1 [M+1] 338.1790, found 338.1792. FTIR (neat): 3279, 2964, 2930, 2871, 1735, 1458, 1365, 1295, 1254, 1214, 1183, 1076, 1022, 884, 847, 760, 702 cm-1.
2-(2-Methyl-propane-2-sulfinylamino)-3-naphthalen-2-yl-hex-3-enoic acid ethyl ester (2c)
1H NMR (400 MHz, CDCl3): 7.83-7.76 (m, 3H), 7.60 (s, 1H), 7.49-7.45 (m, 2H), 7.25 (d, J = 8.6, 1H), 5.90 (t, J = 7.6 Hz, 1H), 4.81 (s, 1H), 4.33 (s, 1H), 4.21-4.13 (m, 2H), 2.02 (qt, J = 7.5 Hz, 2H), 1.20 (s, 9H), 1.18 (t, J = 7.2 Hz, 3H), 0.96 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.3, 136.5, 135.6, 134.9, 133.0, 132.4, 128.0, 127.5, 127.5, 127.4, 126.0, 125.9, 63.1, 61.9, 29.6, 22.7, 22.6, 22.4, 14.0. HRMS: Calcd. for C22H30N1O3S1 [M+1] 388.1946, found 388.1953. FTIR (neat): 3285, 3054, 2961, 2870, 1734, 1597, 1503, 1458, 1365, 1181, 1075, 1019, 859, 749 cm-1.
3-Furan-2-yl-2-(2-methyl-propane-2-sulfinylamino)-hex-3-enoic acid ethyl ester (3c)
1H NMR (400 MHz, CDCl3): 7.36 (t, J = 2.0 Hz, 1H), 6.37-6.36 (m, 1H), 6.30 (d, J = 3.2 Hz, 1H), 5.77 (t, J = 7.2 Hz, 1H), 4.75 (s, 1H), 4.41 (s, 1H), 4.23-4.15 (m, 2H), 2.42 (qt, J = 7.5 Hz, 2H), 1.20 (t, J = 7.0 Hz, 3H), 1.14 (s, 9H), 1.09 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.3, 150.7, 141.3, 137.4, 125.6, 110.7, 109.6, 62.0, 61.8, 29.7, 22.8, 22.4, 14.0, 13.9. HRMS: Calcd. for C16H26N1O4S1 [M+1] 328.1583, found 328.1573. FTIR (neat): 3288, 2966, 2921, 2850, 1735, 1458, 1366, 1261, 1221, 1071, 1025 cm-1.
3-(1H-Indol-2-yl)-2-(2-methyl-propane-2-sulfinylamino)-hex-3-enoic acid ethyl ester (4c)
1H NMR (400 MHz, CDCl3): 8.84 (s, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.14 (td, J = 7.6, 1.1 Hz, 1H), 7.06 (t, J = 7.4 Hz, 1H), 6.45 (d, J = 1.2, 1H), 5.94 (t, J = 7.4 Hz, 1H), 4.78 (d, J = 3.2 Hz, 2H), 4.41 (d, J = 2.4 Hz, 1H), 4.14 (qd, J = 7.2, 1.2 Hz, 2H), 2.45-2.37 (m, 2H), 1.21 (s, 9H), 1.10 (t, J = 7.0 Hz, 3H), 1.08 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): 171.5, 140.1, 136.6, 133.4, 127.9, 126.1, 122.1, 120.3, 119.8, 110.9, 103.9, 62.3, 62.1, 55.9, 22.1, 22.6, 22.5, 14.0, 13.9. HRMS: Calcd. for C20H29N2O3S1 [M+1] 377.1900, found 377.1900. FTIR (neat): 3403, 3276, 2965, 2861, 1732, 1653, 1456, 1366, 1301, 1225, 1054 cm-1. MP: 36 -38 °C.
2-(2-Methyl-propane-2-sulfinylamino)-3-propylidene - octanoic acid ethyl ester (5c)
1H NMR (400 MHz, CDCl3): 5.47 (t, J = 7.2 Hz, 1H), 4.38 (d, J = 4.4 Hz, 1H), 4.25 - 4.16 (m, 1H + 2H), 2.22-2.01 (m, 4H), 1.37-1.25 (m, 6H), 1.25 (s, 9H), 0.98 (t, J = 7.6 Hz, 3H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): 172.2, 134.6, 133.7, 62.3, 61.7, 55.7, 32.0, 28.3, 30.0, 22.6, 22.5, 21.3, 14.1, 14.0, 13.9. HRMS: Calcd. for C17H34N1O3S1 [M+1] 332.2259, found 332.2251. FTIR (neat): 3422, 2959, 2862, 1734, 1638, 1465, 1255, 1077 cm-1.
3-(tert-Butyl-dimethyl-silanyloxymethyl)-2-(2-methyl - propane-2-sulfinylamino)-hex-3-enoicacid ethyl ester (6c)
1H NMR (400 MHz, CDCl3): 5.55 (t, J = 7.4 Hz, 1H), 4.52 (d, J = 4.4 Hz, 1H), 4.27 (d, J = 4.4 Hz, 1H), 4.29-4.09 (m, 2H + 2H), 2.11 (qd, J = 7.5, 2.4 Hz, 2H), 2.78 (t, J = 4.4 Hz, 3H), 1.25 (s, 9H), 0.99 (t, J = 7.4 Hz, 3H), 0.89 (s, 9H), 0.05 (s, 6H). 13C NMR (100 MHz, CDCl3): 171.8, 134.4, 134.2, 61.5, 60.4, 58.2, 55.6, 25.9, 22.6, 21.0, 18.3, 14.0, -5.5. HRMS: Calcd. for C19H40N1O4Si1S1 [M+1] 406.2447, found 406.2448. FTIR (neat): 2957, 2930, 2857, 1736, 1472, 1389, 1364, 1253, 1204, 1082, 849, 777 cm-1.
3-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-2-(2-methy-propane-2-sulfinylamino)-hex-3-enoic acid ethyl ester (7c)
1H NMR (400 MHz, CDCl3): 5.51 (t, J = 7.2 Hz, 1H), 4.38 (d, J = 5.2 Hz, 1H), 4.24 (d, J = 5.2 Hz, 1H), 4.22-4.11 (m, 2H), 3.60-3.48 (m, 2H), 2.36-2.21 (m, 2H), 2.07 (qt, J = 7.5 Hz, 2H), 1.24 (t, J = 7.2 Hz, 3H), 1.22 (s, 9H), 0.95 (t, J = 7.6 Hz, 3H), 0.85 (s, 9H), 0.01 (s, 6H). 13C NMR (100 MHz, CDCl3): 171.9, 135.6, 130.9, 62.6, 61.9, 61.8, 55.7, 31.7, 25.9, 22.6, 21.4, 18.3, 14.0, 13.9, -5.3. HRMS: Calcd. for C20H40N1O4Si1S1 [M+1] 418.2447, found 418.2447. FTIR (neat): 2958, 2857, 1736, 1472, 1364, 1255, 1188, 1084, 836, 776 cm-1.
3-(tert-Butoxycarbonylamino-methyl)-2-(2-methyl-propane-2-sulfinylamino)-hex-3-enoic acid ethyl ester (8c)
1H NMR (400 MHz, CDCl3): 5.71 (t, J = 7.2 Hz, 1H), 4.55 (s, 1H), 4.46 (d, J = 2.8 Hz, 1H), 4.38 (s, 1H), 4.22 (q, J = 7.1, 2H), 3.81 (d, J = 5.2, 2H), 2.19 (qt, J = 7.6, 2H), 1.42 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 1.26 (s, 9H), 1.02 (t, J = 7.6, 3H). 13C NMR (100 MHz, CDCl3): 171.5, 155.4, 138.5, 130.8, 79.3, 62.2, 61.6, 55.7, 37.2, 29.7, 28.4, 22.6, 21.3, 14.0, 13.9. HRMS: Calcd. for C18H35N2O5S1 [M+1] 391.2267, found 391.2278. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
Representative Procedure: the Reductive Coupling of 1,3-Diynes and N-(2,4,6-triisopropyl) benzenesulfinyl iminoacetate
To a solution of N-(2,4,6-triisopropyl) benzenesulfinyl iminoacetate (70.3 mg, 0.2 mmol, 100 mol%) and 1,3-diyne 9a (56.1 mg, 0.4 mmol, 200 mol%) in DCM (2 mL, 0.1M) at ambient temperature was added Rh(COD)2OTf (4.7 mg, 0.01 mmol, 5 mol%) and BIPHEP (5.3 mg, 0.01 mmol, 5 mol%). The system was purged with argon gas followed by hydrogen gas. The reaction was allowed to stir at ambient temperature under 1 atm of hydrogen until complete consumption of substrate, at which point the reaction mixture was evaporated onto silica gel and the product purified by silica gel chromatography.
3-Phenyl-2-(2,4,6-triisopropyl-benzenesulfinylamino) - hept-3-en-5-ynoic acid ethyl ester (9b)
1H NMR (400 MHz, CDCl3): 7.44-7.41 (m, 2H), 7.33-7.27 (m, 3H), 7.02 (s, 2H), 5.88 (qd, J = 2.4, 0.4 Hz, 1H), 4.94 (d, J = 8.0 Hz, 1H), 4.80 (d, J = 8.4 Hz, 1H), 4.19-4.08 (m, 2H), 3.92 (s,2H), 2.84 (qt, J = 6.9 Hz, 1H), 1.82 (d, J = 2.4, 3H), 1.20 (d, J = 6.8, 6H), 1.18 (d, J = 6.8 Hz, 12H), 1.63 (t, J = 7.2, 3H). 13C NMR (100 MHz, CDCl3): 170.8, 152.1, 148.0, 146.3, 137.4, 136.5, 128.6, 128.1, 128.0, 122.9, 111.9, 91.9, 62.8, 61.8, 34.3, 28.1, 24.3, 24.1, 23.7, 23.7, 13.9, 4.5. HRMS: Calcd. for C30H40N1O3S1 [M+1] 494. 2729, found 494.2736. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
7-(tert-Butyl-dimethyl-silanyloxy)-3-phenyl-2-(2,4,6 - triisopropyl-benzenesulfinylamino)-hept-3-en-5-ynoic acid ethyl ester (10b)
1H NMR (400 MHz, CDCl3): 7.40-7.37 (m, 2H), 7.32-7.26 (m, 3H), 7.02 (s, 2H), 5.94 (t, J = 1.0 Hz, 1H), 4.95 (d, J = 8.8 Hz, 1H), 4.80 (d, J = 8.8 Hz, 1H), 4.28 (d, J = 2.0 Hz, 2H), 4.16-4.10 (m, 2H), 3.91 (s,2H), 2.84 (qt, J = 7.0 Hz, 1H), 1.20 (d, J = 7.2, 6H), 1.17 (d, J = 6.8 Hz, 12H), 1.16 (t, J = 7.2, 3H), 0.83 (s, 9H), -0.017 (s, 6H). 13C NMR (100 MHz, CDCl3): 170.6, 152.1, 148.1, 148.0, 137.3, 136.2, 128.6, 128.3, 128.1, 123.0, 111.0, 93.2, 81.6, 62.8, 61.9, 52.1, 34.3, 28.1, 25.7, 24.3, 24.1, 23.7, 18.2, 13.9, -5.31. HRMS: Calcd. for C36H54N1O4Si1S1 [M+1] 624.3543, found 624.3534. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
7-tert-Butoxycarbonylamino-3-phenyl-2-(2,4,6-triisopropyl-benzenesulfinylamino)-hept-3-en-5-ynoic acid ethyl ester (11b)
1H NMR (400 MHz, CDCl3): 7.40-7.37 (m, 2H), 7.38-7.27 (m, 3H), 7.02 (s, 2H), 5.90 (d, J = 0.8 Hz, 1H), 4.94 (d, J = 8.4 Hz, 1H), 4.82 (d, J = 8.4 Hz, 1H), 4. 49 (s, 1H), 4.19-4.07 (m, 2H), 3.90 - 3.88 (m, 4H), 2.84 (qt, J = 6.9 Hz, 1H), 1.40 (s, 9H), 1.21 (d, J = 7.2, 6H), 1.17 (dd, J = 6.8, 2.0 Hz, 12H), 1.15 (t, J = 7.2, 3H). 13C NMR (100 MHz, CDCl3): 170.5, 155.1, 152.1, 148.4, 148.1, 137.3, 136.2, 128.5, 128.4, 128.1, 123.0, 110.8, 90.9, 80.2, 79.8, 62.9, 62.0, 34.3, 31.2, 28.3, 28.2, 24.3, 24.1, 23.7, 23.7, 13.9. HRMS: Calcd. for C35H48N2O5S1 [M+1] 608.3284, found 608.3283. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
3-Furan-2-yl-2-(2,4,6-triisopropyl-benzenesulfinyl amino)-hept-3-en-5-ynoic acid ethyl ester (12b)
1H NMR (400 MHz, CDCl3): 7.29 (d, J = 2.0, 1H), 7.21 (d, J = 3.2, 1H), 7.05 (s, 2H), 6.44 (q, J = 1.7, 1H), 5.68 (q, J = 2.7, 1H), 5.21 (d, J = 9.2 Hz, 1H), 4.93 (d, J = 9.2 Hz, 1H), 4.14 (q, J = 7.1 Hz, 2H), 3.96 (s, 2H), 2.87 (qt, J = 6.9 Hz, 1H), 2.09 (d, J = 2.8, 3H), 1.24 - 1.19 (m, 18H), 1.14 (t, J = 7.0, 3H). 13C NMR (100 MHz, CDCl3): 170.6, 151.9, 150.8, 147.9, 141.4, 137.9, 135.4, 122.9, 111.7, 111.3, 107.9, 96.4, 78.1, 61.9, 61.8, 34.3, 28.2, 24.3, 24.1, 23.8, 23.7, 13.9, 4.94. HRMS: Calcd. for C28H38N1O4S1 [M+1] 484.2522, found 484.2504. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
7-Hydroxy-3-hydroxymethyl-2-(2,4,6-triisopropyl-benzenesulfinylamino)-hept-3-en-5-ynoic acid ethyl ester (13b)
1H NMR (400 MHz, CDCl3): 7.06 (s, 2H), 5.72 (s, 1H), 5.32 (d, J = 8.4 Hz, 1H), 4.75 (d, J = 8.4 Hz, 1H), 4.59 (d, J = 14.4 Hz, 1H), 4.45 (d, J = 14.0 Hz, 1H), 4.41 (d, J = 0.8 Hz, 2H), 4.23-4.15 (m, 2H), 3.94 (s, 2H), 3.50 (S, 1H), 2.89 - 2.82 (m, 1H), 2.31 (s, 1H), 1.31 (d, J = 6.8, 6H), 1.24 (t, J = 7.2, 3H), 1.22 (d, J = 6.8 Hz, 12H),. 13C NMR (100 MHz, CDCl3): 170.3, 152.4, 149.1, 147.7, 137.1, 123.1, 111.5, 95.5, 80.6, 62.3, 61.8, 60.4, 51.3, 34.3, 28.5, 24.4, 24.1, 23.7, 14.0. HRMS: Calcd. for C25H38N1O5S1 [M+1] 464. 2471, found: 464.2472. FTIR (neat): 3301, 2975, 2927, 1738, 1713, 1509, 1459, 1391, 1360, 1248, 1174, 1075 cm-1.
deuterio-3-Phenyl-2-(2,4,6-triisopropylbenzene sulfinylamino)-hept-3-en-5-ynoic acid ethyl ester (deuterio -9b)
1H NMR (400 MHz, CDCl3): 7.45 - 7.43 (m, 2H), 7.34-7.28 (m, 3H), 7.03 (s, 2H), 4.96 (d, J = 8.0 Hz, 1H), 4.81 (d, J = 8.0 Hz, 1H), 4.19-4.09 (m, 2H), 3.92 (br, 2H), 2.89-2.82 (m, 1H), 1.83 (s, 3H), 1.25-1.16 (m, 2H). 13C NMR (100 MHz, CDCl3): 170.8, 152.0, 148.0, 146.1, 137.3, 136.4, 128.5, 128.1, 128.0, 122.9, 91.8, 62.7, 61.8, 34.2, 28.1, 24.2, 24.1, 23.7, 13.9, 4.5. HRMS: Calcd. for C30H39D1N1O3S1 [M+1] 495.2792, found 495.2785. FTIR (neat): 2960, 2868, 1739, 1597, 1462, 1363, 1257, 1189, 1094, 1027, 879, 697 cm-1.
Representative Procedure for the Exhaustive Hydrogenation of Dienes and Enynes
To a solution of diene 14b (198.9 mg, 0.6 mmol, 100 mol%) in DCM (6 mL, 0.1M) at ambient temperature was added Ir(COD)(Pyr)[P(c-Hex)3] PF6 (24.2 mg, 0.03 mmol, 5 mol%). The system was purged with argon gas followed by hydrogen gas. The reaction was allowed to stir at ambient temperature under 1 atm of hydrogen until complete consumption of substrate, at which point the reaction mixture was evaporated onto silica gel and the product purified by silica gel chromatography.
2-tert-Butoxycarbonylamino-3-phenyl-hexa-3,5-dienoic acid ethyl ester (14b)
1H NMR (400 MHz, CDCl3): 7.17-7.26 (m, 3H), 7.17 (s, J = 7.2 Hz, 2H), 6.39 (d, J = 11.2 Hz, 1H), 6.23 (dt, J = 16.8, 10.5 Hz, 1H), 5.34 (d, J = 1.6 Hz, 1H), 5.30 (d, J = 1.6 Hz, 1H), 5.10 (d, J = 10.0 Hz, 1H), 5.06 (d, J = 8.0 Hz, 1H), 4.14 (m, 2H), 1.43 (s, 9H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): 170.5, 154.7, 138.3, 137.0, 133.3, 131.0, 129.2, 128.2, 127.7, 119.6, 79.9, 61.6, 59.7, 28.2, 13.7. HRMS: Calcd. for C19H26N1O4 [M+1] 332.1862, found 332.1862. FTIR (neat): 3439, 2978, 2933, 1740, 1717, 1492, 1367, 1323, 1248, 1162, 1058, 1026, 912, 865, 777, 704 cm-1.
2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-phenyl - hexa-3,5-dienoic acid ethyl ester (15b)
1H NMR (400 MHz, CDCl3): 7.77 (d, J = 7.6 Hz, 2H), 7.60-7.55 (m, 2H), 7.42-7.29 (m, 7H), 7.20 (d, J = 6.8 Hz, 2H), 6.44 (d, J = 10.8 Hz, 1H), 6.23 (dt, J = 17.2, 10.5 Hz, 1H), 5.68 (d, J = 8.0 Hz, 1H), 5.36 (d, J = 17.2 Hz, 1H), 5.15 (d, J = 7.2 Hz, 1H), 5.14 (d, J = 10.4 Hz, 1H), 4.44 (dd, J = 10.2, 7.0 Hz, 1H), 4.26 (dd, J = 10.4, 6.8 Hz, 1H), 4.25 - 4.17 (m, 3H), 1.21 (t, J = 7.2, 3H). 13C NMR (100 MHz, CDCl3): 170.2, 155.2, 143.7, 143.6, 141.2, 138.0, 136.9, 133.2, 131.2, 129.2, 128.2, 127.8, 127.6, 127.0, 125.1, 125.0, 120.0, 119.9, 67.0, 61.8, 60.0, 47.1, 14.0. HRMS: Calcd. for C29H28N1O4 [M+1] 454.2018, found 454.2015. FTIR (neat): 3344, 2979, 1720, 1498, 1449, 1321, 1194, 1047, 912, 758, 740, 703 cm-1.
2-tert-Butoxycarbonylamino-3-phenyl-hept-3-en-5-ynoic acid ethyl ester (16b)
1H NMR (400 MHz, CDCl3): 7.47-7.36 (m, 2H), 7.37-7.27 (m, 3H), 5.85 (d, J = 2.0 Hz, 1H), 5.36 (d, J = 7.2 Hz, 1H), 5.12 (d, J = 7.6 Hz, 1H), 4.19-4.07 (m, 2H), 1.84 (d, J = 2.4, 3H), 1.42 (s, 9H), 1.15 (t, J = 6.8, 3H). 13C NMR (100 MHz, CDCl3): 170.4, 154.6, 146.1, 136.9, 128.3, 127.9, 110.9, 91.4, 80.0, 61.7, 58.7, 28.2, 13.9, 4.5. HRMS: Calcd. for C20H26N1O4 [M+1] 344.1862, found 344.1861. FTIR (neat): 3375, 2978, 2932, 1740, 1717, 1495, 1367, 1325, 1247, 1164, 1054, 1026, 913, 865, 744, 699 cm-1.
2-tert-Butoxycarbonylamino-3-phenyl-hexanoic acid ethyl ester (14d)
1H NMR (300MHz, CDCl3) Major: 7.33-7.24 (m, 3H), 7.18-7.15 (m, 2H), 5.06 (d, J = 9.3 Hz, 1H), 4.47 (t, J = 8.1 Hz, 1H), 3.98 (q, J = 7.1 Hz, 2H), 2.93 (q, J = 7.4 Hz, 1H), 1.78 (q, J = 7.5 Hz, 2H), 1.45 (s, 9H), 1.27-1.12 (m, 2H), 1.05 (t, J = 7.0, 3H), 0.87 (t, J = 7.4 Hz, 3H). Minor: 7.30 -7.11 (m, 3H), 7.11 - 7.09 (m, 2H), 4.77 (d, J = 8.7 Hz, 1H), 4.55 (dd, J = 9.3, 5.1 Hz, 1H), 4.11 (q, J = 7.0 Hz, 2H), 3.16 (dd, J = 16.2, 7.5 Hz, 1H), 1.72 (q, J = 7.9 Hz, 2H), 1.39 (s, 9H), 1.24 - 1.19 (m, 5H), 0.85 (t, J = 7.2 Hz, 3H). 13C NMR (100MHz, CDCl3) Major: 171.8, 155.2, 139.9, 128.4, 128.3, 127.0, 79.8, 60.9, 58.4, 49.1, 33.3, 28.3, 20.5, 13.9, 13.8. HRMS: Calcd. for C19H30N1O4 [M+1] 336.2175, found 336.2175. FTIR (mixture, neat): 3442, 3558, 2960, 2932, 2871, 1718, 1496, 1454, 1391, 1367, 1340, 1253, 1171, 1048, 1026, 863, 776, 757, 701 cm-1.
2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-phenyl - hexanoic acid ethyl ester (15d)
1H NMR (400MHz, CDCl3) Major: 7.78 (d, J = 7.6 Hz,2H), 7.61 (d, J = 7.2 Hz, 2H), 7.42 (t, J = 7.6 Hz, 2H), 7.35-7.24 (m, 5H), 7.14 (d, J = 7.6 Hz, 2H), 5.33 (d, J = 9.2 Hz, 1H), 4.55 (t, J = 8.2 Hz, 1H), 4.47 (dd, J = 10.4, 7.2 Hz, 1H), 4.36 (dd, J = 10.4, 7.2 Hz, 1H), 4.23 (t, J = 6.8 Hz, 1H), 4.00 (q, J = 7.2 Hz, 2H), 2.96 (q, J = 7.3 Hz, 1H), 1.80 (q, J = 7.6 Hz, 2H), 1.24-1.15 (m, 2H), 1.05 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). Minor: 7.77 (d, J = 7.6 Hz,2H), 7.56 (t, J = 8.4 Hz, 2H), 7.41 (td, J = 7.3, 1.9 Hz, 2H), 7.34-7.24 (m, 5H), 7.10 (d, J = 6.8 Hz, 2H), 5.04 (d, J = 9.6 Hz, 1H), 4.66 (q J = 4.7 Hz, 1H), 4.45 (dd, J = 10.8, 7.2 Hz, 1H), 4.34 (dd, J = 10.8, 6.8 Hz, 1H), 4.22 (t, J = 7.0 Hz, 1H), 4.15 (q, J = 7.2 Hz, 2H), 3.23 (q, J = 9.8 Hz, 1H), 1.73 (q, J = 11.4 Hz, 2H), 1.30-1.22 (m, 2H), 1.26 (t, J = 7.0 Hz, 3H), 0.90 (t, J = 7.0 Hz, 3H). 13C NMR (100MHz, CDCl3) Major: 171.4, 155.7, 143.9, 143.7, 141.3, 139.5, 128.4, 128.3, 127.7, 127.1, 127.0, 125.1, 125.0, 119.9, 66.9, 61.1, 58.8, 49.1, 47.2, 33.2, 20.5, 13.9, 13.8. HRMS: Calcd. for C29H32N1O4 [M+1] 458.2331, found 458.2336. FTIR (neat): 3442, 3558, 2960, 2932, 2871, 1718, 1496, 1454, 1391, 1367, 1340, 1253, 1171, 1048, 1026, 863, 776, 757, 701 cm-1.
2-tert-Butoxycarbonylamino-3-phenyl-heptanoic acid ethyl ester (16d)
1H NMR (400 MHz, CDCl3): 7.31-7.11 (m, 5H), 5.04 and 4.78 (major: d, J = 9.2 Hz, minor: d, J = 8.8 Hz, 1H), 4.57 and 4.45 (minor: dd, J = 9.6, 5.2 Hz, major: t, J = 8.0 Hz, 1H), 4.12 and 3.97 (minor: q, J = 7.2 Hz, major: q, J = 7.2 Hz, 2H), 3.18-3.15 and 2.91-2.86 (minor: m, major: m, 1H), 1.82-1.74 (m, 2H), 1.42 and 1.41 (major: s, minor: s, 9H), 1.38-1.01 (m, 7H), 0.85-0.80 (m, 3H). 13C NMR (100 MHz, CDCl3) Major: 171.7, 155.1, 139.9, 128.4, 128.2, 126.9, 79.7, 60.8, 58.4, 49.3, 30.7, 29.5, 28.2, 22.5, 13.8. Minor: 171.7, 155.6, 139.4, 128.4, 128.2, 127.1, 79.7, 61.0, 57.6, 47.8, 31.1, 29.4, 28.2, 22.5, 14.1. HRMS: Calcd. for C20H32N1O4 [M+1] 350.2331, found 350.2329. FTIR (neat): 3359, 2958, 2932, 2870, 1718, 1496, 1454, 1367, 1250, 1171, 1055, 1027, 864, 700 cm-1.
Procedure for Cross-Metathesis Reaction with Diene 14b and 1,4-Diacetoxy-cis-2-butene. 7-Acetoxy-2-tert-butoxycarbonylamino-3-phenyl-hepta-3,5-dienoic acid ethyl ester (14e)
To a solution of Grubbs’ catalyst (17.6 mg, 0.021 mmol, 5 mol%) in DCM (2.1 mL, 0.2 M) was added diene 14b (139.4 mg, 0.42 mmol, 100 mol%) and 1,4-diacetoxy-cis-2-butene (215 mg, 1.25 mmol, 300 mol%), and the solution stirred for 12 h at 40 °C. The volatiles were removed by rotary evaporation, and the residue was purified by silica flash chromatography to provide the title compounds as a yellow oil. 1H NMR (400 MHz, CDCl3): 7.35-7.30 (m, 3H), 7.15 (d, J = 6.8 Hz, 2H), 6.63 and 6.37 (minor: d, J = 11.6 Hz, major: d, J = 10.8 Hz, 1H), 6.12 and 6.01 (major: t, J = 13.0 Hz, minor: t, J = 11.6 Hz, 1H), 5.85 and 5.53-5.47 (major: dt, J = 15.2, 6.4 Hz, minor: m, 1H), 5.33 and 5.13 (major: d, J = 7.2 Hz, minor: m, 1H), 5.03 and 4.89 (major: d, J =7.6 Hz, minor: m, 1H), 4.77 and 4.48 (minor: d, J = 6.8 Hz, major: d, J = 6.0 Hz, 2H), 4.12 (q, J = 7.1 Hz, 2H), 2.06 and 1.99 (minor: s, major: s, 3H), 1.41 (s, 9H), 1.16 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) Major: 190.6, 154.6, 139.4, 136.7, 130.4, 129.3, 129.2, 129.1, 129.0, 128.2, 127.8, 79.9, 64.6, 61.7, 59.7, 28.2, 20.8, 13.9. HRMS: Calcd. for C22H30N1O6 [M+1] 404.2073, found 404.2069. FTIR (neat): 3375, 3374, 2978, 2923, 1741, 1715, 1493, 1368, 1325, 1230, 1161, 1056, 1025, 976, 865, 777, 704.
Supplementary Material
Table 2.
Hydrogen-mediated reductive coupling of 1,3-diynes 9a-13a and ethyl N-(2,4,6-triisopropylbenzenesulfinyl)iminoacetatea
| Entry | Substrate | Regioisomeric Coupling Products | |
|---|---|---|---|
| 1 |  |
 |
 |
| 2 |  |
 |
 |
| 3 |  |
 |
 |
| 4 |  |
 |
 |
| 5 |  |
 |
N/A |
The reductive coupling of 9a-12a was performed at ambient temperature, in accordance with the reaction conditions cited in Scheme 2. The reductive coupling of 13a was performed at 35 °C in DCM-THF (2:1). The reductive couplings were complete in less than 2 hours. See experimental section for detailed reaction conditions.
Acknowledgment
Acknowledgment is made to the Research Corporation Cottrell Scholar Program (CS0927), the Sloan Foundation, the Dreyfus Foundation, Eli Lilly, Johnson & Johnson, Merck, Boehringer-Ingelheim and the NIH-NIGMS (RO1-GM69445) for partial support of this research. This work is dedicated to the memory of Henry Rapoport.
References
- (1)(a).Alkenes:Kablaoui NM, Buchwald SL. J. Am. Chem. Soc. 1995;117:6785.Crowe WE, Rachita MJ. J. Am. Chem. Soc. 1995;117:6787.Kablaoui NM, Buchwald SL. J. Am. Chem. Soc. 1996;118:3182.
- (2)(a).Alkynes and Dienes (review articles):Montgomery J. Acc. Chem. Res. 2000;33:467. doi: 10.1021/ar990095d.Montgomery J, Amarashinghe KKD, Chowdhury SK, Oblinger E, Seo J, Savchenko AV. Pure Appl. Chem. 2002;74:129.Ikeda S-I. Angew. Chem. Int. Ed. 2003;42:5120. doi: 10.1002/anie.200301673.Montgomery J. Angew. Chem. Int. Ed. 2004;43:3890. doi: 10.1002/anie.200300634.
- (3)(a).Allenes:Chevliakov MV, Montgomery J. J. Am. Chem. Soc. 1999;121:11139.Montgomery J, Song M. Org. Lett. 2002;4:4009. doi: 10.1021/ol026670m.Amarasinghe KKD, Montgomery J. J. Am. Chem. Soc. 2002;124:9366. doi: 10.1021/ja027148y.
- (4)(a).Enones (review articles):Huddleston RR, Krische MJ. Synlett. 2003:12.Jang H-Y, Krische MJ. Eur. J. Org. Chem. 2004:3953. doi: 10.1021/jo030310a.
- (5).1,3-Enynes:Miller KM, Luanphaisarnnont T, Molinaro C, Jamison TF. J. Am. Chem. Soc. 2004;126:4130. doi: 10.1021/ja0491735.
- (6)(a).For hydrogen-mediated C-C bond formations developed in our lab, see:Jang H-Y, Huddleston RR, Krische MJ. J. Am. Chem. Soc. 2002;124:15156. doi: 10.1021/ja021163l.Huddleston RR, Krische MJ. Org. Lett. 2003;5:1143. doi: 10.1021/ol0300219.Koech PK, Krische MJ. Org. Lett. 2004;6:691. doi: 10.1021/ol030136c.Marriner GA, Garner SA, Jang H-Y, Krische MJ. J. Org. Chem. 2004;69:1380. doi: 10.1021/jo030310a.Jang H-Y, Huddleston RR, Krische M. Angew. Chem. Int. Ed. 2003;42:4074. doi: 10.1002/anie.200351986.Jang H-Y, Huddleston RR, Krische MJ. J. Am. Chem. Soc. 2004;126:4664. doi: 10.1021/ja0316566.Huddleston RR, Jang H-Y, Krische MJ. J. Am. Chem. Soc. 2003;125:11488. doi: 10.1021/ja030415v.Jang H-Y, Krische MJ. J. Am. Chem. Soc. 2004;126:7875. doi: 10.1021/ja048498i.Jang H-Y, Hughes FW, Gong H, Zhang J, Brodbelt JS, Krische M. J. Am. Chem. Soc. 2005;127:6174. doi: 10.1021/ja042645v.
- (7).For a review, see:Jang H-Y, Krische MJ. Acc. Chem. Res. 2004;37:653. doi: 10.1021/ar020108e.
- (8)(a).Prior to our work, two isolated studies on hydrogen-mediated C-C bond formation under CO-free conditions were reported:Molander GA, Hoberg JO. J. Am. Chem. Soc. 1992;114:3123.Kokubo K, Miura M, Nomura M. Organometallics. 1995;14:4521.
- (9)(a).Products of C-C bond formation have been observed as side products in catalytic hydrogenation on rare occasion:Moyes RB, Walker DW, Wells PB, Whan DA, Irvine EA. Special Pub. Royal. Soc. Chem. 1992;114:207.Bianchini C, Meli A, Peruzzini M, Vizzi F, Zanobini F, Frediani P. Organometallics. 1989;8:2080.
- (10)(a).Patel SJ, Jamison TF. Angew. Chem., Int. Ed. 2003;42:1364. doi: 10.1002/anie.200390349. [DOI] [PubMed] [Google Scholar]; (b) Patel SJ, Jamison TF. Angew. Chem., Int. Ed. 2004;43:3941. doi: 10.1002/anie.200460044. [DOI] [PubMed] [Google Scholar]
- (11)(a).For recent reviews encompassing the use of N-tert-butanesulfinyl imines in asymmetric synthesis, see:Zhou P, Chen B-C, Davis F. Tetrahedron. 2004;60:8003.Ellman JA, Owens TD, Tang TP. Acc. Chem. Res. 2002;35:984. doi: 10.1021/ar020066u.
- (12)(a).For recent examples of the use of N-sulfinyl imines in asymmetric synthesis, see:Evans JW, Fierman MB, Miller SJ, Ellman JA. J. Am. Chem. Soc. 2004;126:8134. doi: 10.1021/ja047845l.Davis FA, Ramachandar T, Liu H. Org. Lett. 2004;6:3393. doi: 10.1021/ol0485971.Han Z, Krishnamurthy D, Grover P, Fang QK, Senanayake CH. J. Am. Chem. Soc. 2002;124:7880. doi: 10.1021/ja0200692.
- (13).For a review encompassing the asymmetric synthesis of amino acids through additions to N-tert-butanesulfinyl imines, see:Davis F, Chen B-C. Chem. Soc. Rev. 1998;27:13.
- (14).The partial hydrogenation of conjugated dienes under similar conditions has been reported:Schrock RR, Osborn JA. J. Am. Chem. Soc. 1976;98:4450.
- (15).For the exhaustive hydrogenation of conjugated dienes catalyzed by iridium, see:Cui X, Burgess K. J. Am. Chem. Soc. 2003;125:14212. doi: 10.1021/ja037653a.0and references therein.
- (16).Funk TW, Efskind J, Grubbs RH. Org. Lett. 2005;7:187. doi: 10.1021/ol047929z. [DOI] [PubMed] [Google Scholar]
- (17)(a).For σ-bond metathesis involving Rh(III) intermediates, see:Hartwig JF, Cook KS, Hapke M, Incarvito CD, Fan Y, Webster CE, Hall MB. J. Am. Chem. Soc. 2005;127:2538. doi: 10.1021/ja045090c.Liu C, Widenhoefer RA. Organometallics. 2002;21:5666.Hutschka F, Dedieu A, Leitner W. Angew. Chem. Int. Ed. Engl. 1995;34:1742.
- (18)(a).Dewar MJS. Bull. Soc. Chim. Fr. 1951;18:C71. [Google Scholar]; (b) Chatt J, Duncanson LA. J. Chem. Soc. 1953:2939. [Google Scholar]; (c) Dewar MJS, Ford GP. J. Am. Chem. Soc. 1979;101:783. [Google Scholar]
- (19).The nucleophilic character of alkenes and alkynes bound to low valent titanium constitutes the basis of legion reductive C-C bond formations. For a review, see:Sato F, Urabe H, Okamoto S. Chem. Rev. 2000;100:2835. doi: 10.1021/cr990277l.
- (20).Siebenlist R, Frühauf H-W, Vrieze K, Kooijman H, Smeets WJJ, Spek AL. Organometallics. 2000;19:3016.and references therein. See also:Taggi AE, Hafez AM, Lectka T. Acc. Chem. Res. 2003;36:10. doi: 10.1021/ar020137p.
- (21)(a).For vinyl sulfoxides, a C=C-S-O dihedral angle of 0° is strongly preferred:Tietze LF, Schuffenhauer A, Schreiner PR. J. Am. Chem. Soc. 1998;120:7952.Kahn D, Hehre WJ. J. Am. Chem. Soc. 1986;108:7399.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


