The findings of this study suggest that mutant NPM1, and more specifically the genotype mutant NPM1 without FLT3-ITD, is a predictive factor for response to all-trans retinoic acid given as an adjunct to intensive chemotherapy in older patients with acute myeloid leukemia. See related perspective article on page 10.
Keywords: acute myeloid leukemia, all-trans retinoic acid, nucleophosmin-1 mutation, predictive factor
Abstract
Background
In a previous randomized trial, AML HD98B, we showed that administration of all-trans retinoic acid in addition to intensive chemotherapy improved the outcome of older patients with acute myeloid leukemia. The objectives of this study were to evaluate the prognostic impact of gene mutations and to identify predictive genetic factors for the all-trans retinoic acid treatment effect.
Design and Methods
Data from mutation analyses of the NPM1, CEBPA, FLT3, and MLL genes were correlated with outcome in patients 61 years and older treated within the AML HD98B trial.
Results
The frequencies of mutations were: NPM1, 23%; CEBPA, 8.5% (analysis restricted to patients with a normal karyotype); FLT3 internal tandem duplications (ITD), 17%; FLT3 tyrosine kinase domain mutations, 5%; and MLL partial tandem duplications, 4.5%. The genotype mutant NPM1 was positively and adverse cytogenetics as well as higher white blood cell count negatively correlated with achievement of complete remission. In Cox regression analysis, a significant interaction between the genotype mutant NPM1 without FLT3-ITD and treatment with all-trans retinoic acid was identified, in that the beneficial effect of all-trans retinoic acid on relapse-free and overall survival was restricted to this subgroup of patients. Other significant factors for survival were age, adverse cytogenetics, and logarithm of white cell count.
Conclusions
In elderly patients with acute myeloid leukemia, NPM1 mutations are associated with achievement of complete remission, and the genotype ‘mutant NPM1 without FLT3-ITD’ appears to be a predictive marker for response to all-trans retinoic acid given as an adjunct to intensive chemotherapy (ClinicalTrials.gov Identifier: NCT00151242).
Introduction
There has been a long-standing interest in the clinical use of all-trans retinoic acid (ATRA) in the treatment of acute myeloid leukemia (AML) subtypes other than acute promyelocytic leukemia (APL). Based on promising in vitro data,1–6 several clinical trials evaluated ATRA in combination with intensive chemotherapy in non-APL AML.7–12 Initial encouraging data came from a phase II trial combining low-dose cytarabine with ATRA in 33 patients ineligible for intensive therapy.7 However, the results from subsequent randomized studies have been contradictory, with the majority reporting negative results. Estey et al. studied 215 patients older than 71 years with high-risk myelodysplastic syndrome or AML.8 Although no effect of ATRA could be shown in multivariable analysis, univariate analysis revealed significantly better overall survival in the treatment arms containing ATRA. The British Medical Research Council (MRC) performed three randomized trials, one in younger patients receiving first-line treatment (MRC AML12, n=1097),9 one in medically unfit patients (MRC AML14, n=207),10 and one in high-risk, refractory, or relapsed patients (MRC AML-HR, n=362).11 In none of these trials was there a significant effect of ATRA on any end-point analyzed. In contrast, in our trial of AML patients aged 61 years and older (AML HD98B, n=242), patients randomized to the ATRA arm had a significantly higher complete remission rate and better event-free and overall survival.12 We hypothesized that the beneficial effect of ATRA may be restricted to a specific biological subgroup of AML.
Recently, somatic mutations in the NPM1, CEBPA, FLT3, and MLL genes have been identified in AML. In younger adult patients, these mutations have been shown to be of prognostic and predictive relevance.13,14 In particular, the genotypes ‘mutant NPM1 without FLT3-ITD’ and ‘mutant CEBPA’ have emerged as significant factors for achievement of complete remission as well as better relapse-free survival and overall survival. To date, little is known about the impact of these gene mutations in older patients with AML.
The objectives of this study were to evaluate the association of mutations in NPM1, CEBPA, FLT3, and MLL with clinical outcomes in the patients who had been entered into the treatment trial AML HD98B of the German-Austrian AML Study Group (AMLSG),12,15,16 and to identify predictive factors for the beneficial treatment effect of ATRA.
Design and Methods
Patients and treatment
Between March 1998 and July 2004, 377 patients were prospectively enrolled into the AMLSG AML HD98B treatment trial.12,15,16
Patients 61 years or older with de novo AML or refractory anemia with excess of blasts in transformation as defined by the French–American–British classification system,17 secondary AML with a preceding history of myelodysplasia of at least 3 months before the diagnosis of AML, or therapy-related AML following treatment of a primary malignancy were eligible for the trial. Patients with APL were excluded. Details of the treatment plan have already been published.12,16 Briefly, patients received two induction cycles of idarubicin, standard-dose cytarabine, and etoposide with or without ATRA, followed by one consolidation cycle of intermediate-dose cytarabine and mitoxantrone with or without ATRA. During induction therapy, ATRA was given at a dose of 45 mg/m2 on days 3 through 5, and at 15 mg/m2 on days 6 through 28. During first consolidation, ATRA was given at 15 mg/m2 on days 3 through 28. For second consolidation therapy, patients were randomized to either intensive therapy with idarubicin and etoposide or 12 monthly courses of outpatient maintenance therapy with the same agents given orally.16 Allogeneic hematopoietic stem-cell transplantation was allowed for patients with an HLA-identical family donor at the discretion of the local investigator. For conditioning, a combination of fludarabine, cyclophosphamide, idarubicin, and etoposide was recommended.18
Following a pilot phase that included 19 patients, 242 patients were randomized for ATRA, 122 into the experimental arm and 120 into the standard arm. The planned interim analysis in 2001 revealed a trend for a better complete remission rate in the experimental arm. However, the difference was not statistically significant and, according to protocol, randomization for ATRA was stopped. An additional 116 patients were assigned to the standard arm to achieve the sample size required for second randomization. A diagram summarizing the randomized patient population is provided in the original report.12
Written informed consent was obtained from all patients at study entry. The study was approved by the local Ethics Review Committees of the participating institutions.
Cytogenetic and molecular genetic analysis
Leukemia samples were studied centrally in the Laboratory for Cytogenetic and Molecular Diagnostics at the University Hospital of Ulm. Data from conventional chromosome analysis were available for 316 of the 377 (84%) patients and have been published elsewhere.15 To improve diagnostic accuracy, all specimens were also analyzed by fluorescence in situ hybridization using a DNA probe set for the detection of the following AML-associated cytogenetic aberrations: inv(3)/t(3;3), t(8;21), t(9;22), t(11q23), t(15;17), inv(16)/t(16;16), +4q, del(5q), del(7q), +8q, +11q, abn(12p), del(13q)/+13q, del(17p), del(20q), +21q, +22q, and del(Xq).19
For the present study, diagnostic samples were analyzed for mutations in the NPM1, CEBPA, FLT3 (internal tandem duplication [FLT3-ITD] and tyrosine kinase domain mutations at codons D835 and I836 [FLT3-TKD]), and MLL (partial tandem duplication [MLL-PTD]) genes using previously reported methods.14,20–23 CEBPA mutation analysis was restricted to patients with a normal karyotype.
Criteria for treatment outcomes
Response to induction therapy was assessed after two courses of chemotherapy. In accordance with standard criteria,24 complete remission was defined as less than 5% bone marrow blasts, an absolute neutrophil count of 1.5×109/L or more, a platelet count of 100×109/L or more, no blasts in the peripheral blood, and no extramedullary leukemia. Therapeutic failures were classified as either refractory disease or early/hypoplastic death (death less than 7 days after completion of the first course of induction therapy/death during the remainder of double induction therapy). Relapse was defined as more than 5% bone marrow blasts unrelated to recovery from the preceding course of chemotherapy or new extramedullary leukemia in patients with previously documented complete remission. End-points for overall survival, measured from the date of study entry, were death (failure) and alive at last follow-up (censored). End-points for relapse-free survival, measured from the date of achievement of complete remission, were death in complete remission or relapse (failure) and alive in complete remission at last follow-up (censored).
Statistical analyses
The median duration of follow-up was calculated according to the method of Korn.25 Analyses in relation to clinical outcome were restricted to the upfront randomized patients according to their randomization. A logistic regression model was used to analyze associations between presenting features as well as treatment with ATRA and achievement of complete remission. The Kaplan-Meier method was used to estimate the distribution of overall survival; confidence interval (CI) estimation was based on the cumulative hazard using Greenwood’s formula for standard error (SE) estimation. Survival distributions were compared using the log-rank test. A Cox model was used to identify prognostic variables. In addition to the molecular markers (NPM1, MLL, FLT3-ITD, and FLT3-TKD mutations), cytogenetics, age, white blood cell count (WBC), platelet count, bone marrow blast count, presence or absence of hepato-spenomegaly, and type of AML were added as explanatory variables. In multivariable models cytogenetics was categorized into three groups, core binding factor (CBF), normal karyotype and all other aberrations into the group of adverse cytogenetics. For multivariable analyses, we performed a missing value imputation in the subset of patients with at least one molecular marker analyzed.26 The frequency of missing data for the single co-variates was below 20%. We estimated missing data for co-variates by using 50 multiple imputations in chained equations incorporating predictive mean matching.26 All statistical analyses were performed with the statistical software environment R, version 2.4.1, using the R package Design, version 2.0–12.27 p values of less than 0.05 were considered to indicate statistical significance.
Results
Molecular markers and baseline characteristics
Molecular markers were analyzed in all available diagnostic peripheral blood and/or bone marrow samples (NPM1, n=252; CEBPA, n=117 [restricted to normal karyotype AML]; FLT3-ITD, n=295; FLT3-TKD, n=279; MLL-PTD, n=250). The mutation status of all four genes (excluding CEBPA) was available for 206 patients (55%), and at least one marker could be analyzed in 302 of the 377 (80%) patients.
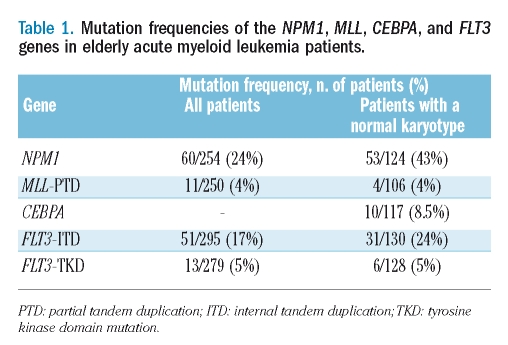
The frequencies of the mutations for all patients and for the subgroup of patients with cytogenetically normal AML are given in Table 1. NPM1 mutations and FLT3-ITD were significantly more frequent in cytogenetically normal AML (p<0.0001 and p=0.005, respectively), whereas no such association was found for FLT3-TKD (p=0.50) or MLL-PTD (p=0.75).
Table 1.
Mutation frequencies of the NPM1, MLL, CEBPA, and FLT3 genes in elderly acute myeloid leukemia patients.
In cytogenetically normal AML, MLL-PTD showed no overlap with NPM1 or CEBPA mutations, whereas three of ten patients with mutant CEBPA showed a concurrent NPM1 mutation. Both types of FLT3 mutations were more frequently associated with mutant NPM1 (ITD, p<0.0001; TKD p=0.04), whereas no such association was present with MLL-PTD. Of ten patients with mutant CEBPA, two had a FLT3-ITD and none had a FLT3-TKD mutation.
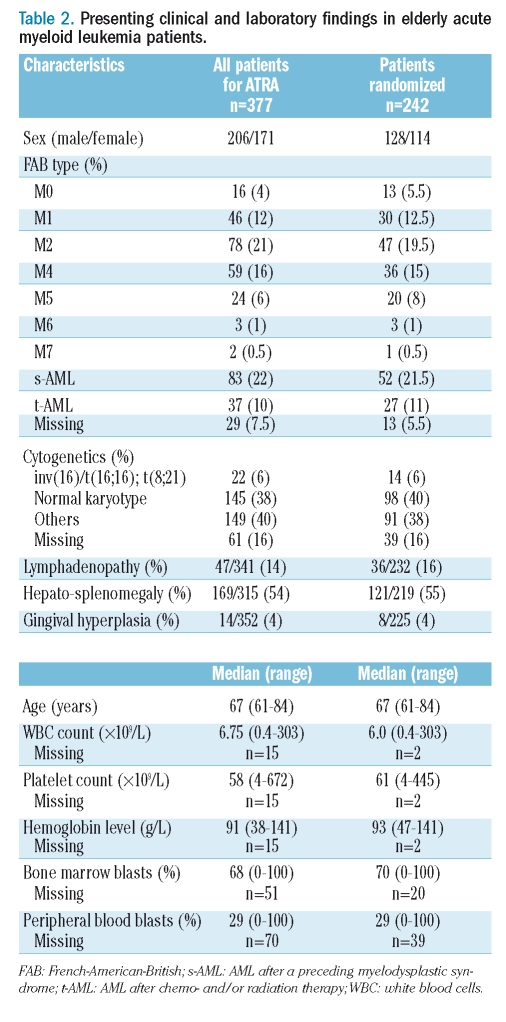
Pretreatment characteristics of all patients and the subgroup of patients randomized for ATRA are given in Table 2.
Table 2.
Presenting clinical and laboratory findings in elderly acute myeloid leukemia patients.
Induction therapy
Of the 242 patients randomized for ATRA, 114 (47%) achieved a complete remission, 92 (38%) had refractory disease, and 36 (15%) died. Logistic regression analysis of patients for whom at least one molecular marker was analyzed (n=206) revealed that mutant NPM1 (odds ratio [OR], 3.17; 95% CI, 1.37–7.35; p=0.02), logarithm of WBC (OR, 0.58; 95% CI, 0.35–0.96; p=0.03) and adverse cytogenetics (OR, 0.46; 95% CI, 0.21–0.99; p=0.05) were significantly associated with achievement of complete remission. The molecular markers FLT3-ITD, FLT3-ITD, and MLL-PTD, clinical characteristics such as age, platelet count, percentage of bone marrow blasts, and type of AML, as well randomization to ATRA had no significant impact.
Survival analyses according to treatment with all-trans retinoic acid
The median follow-up time for survival was 68.5 months. Patients randomized to ATRA had a significantly better relapse-free survival (p=0.006) and overall survival (p=0.003), with 4-year relapse-free survival and overall survival rates of 20.9% (95% CI, 12.5–30.8%) and 10.8% (95% CI, 6.1–17%), respectively, as compared to 4.8% (95% CI, 1.6–10.9%) and 5% (95% CI, 2–10%), respectively, in the standard treatment arm of the study.
Survival analyses according to molecular markers and treatment with all-trans retinoic acid
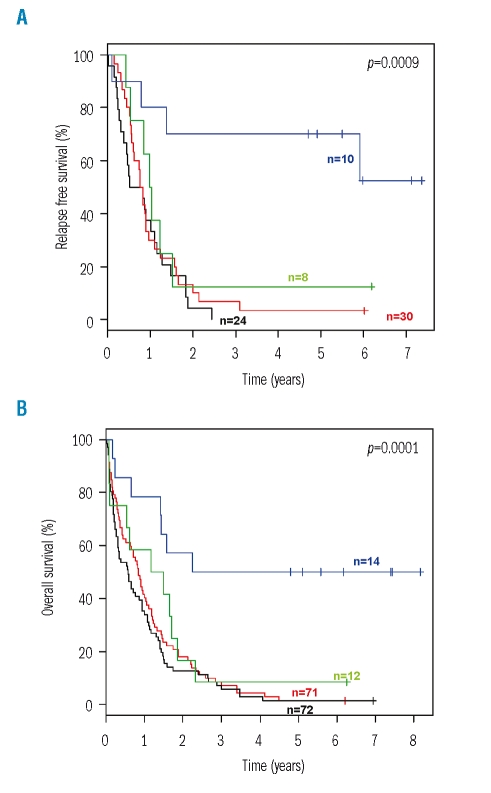
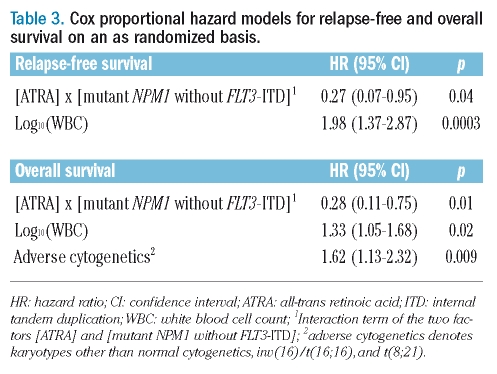
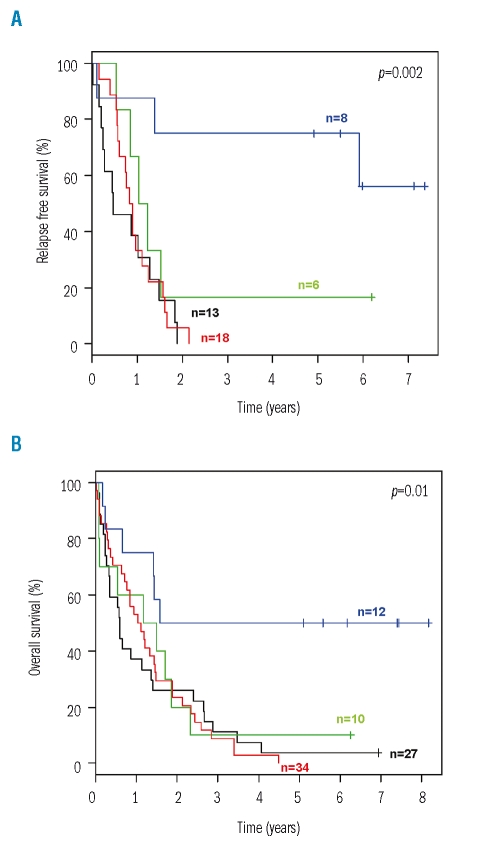
The evaluation of predictive factors for the beneficial treatment effect of ATRA was performed on an intention-to-treat basis. In univariable survival analyses, an interaction was found between genotypes and the effect of randomization to ATRA. A significant difference in both relapse-free and overall survival in favor of the ATRA arm was evident in patients with the genotype ‘mutant NPM1 without FLT3-ITD’, whereas relapse-free and overall survival of patients with all other combinations of these two genetic markers were dismal, irrespective of whether they had been randomized to ATRA or not (Figure 1). For Cox regression analysis, we included an interaction term to account for the observed interaction between genotypes and randomization to ATRA. Cox regression models for relapse-free survival and overall survival, using the genotype ‘mutant NPM1 without FLT3-ITD’ revealed a significant interaction between this marker constellation and randomization to ATRA. The hazard ratios and 95% CI of the significant factors are given in Table 3. Since both NPM1 mutation and FLT3-ITD were significantly associated with cytogenetically normal AML, we also performed a univariable analysis in this subset of patients. Again, a significant difference in both relapse-free and overall survival in favor of the ATRA arm was evident in patients with the genotype ‘mutant NPM1 without FLT3-ITD’ (Figure 2).
Figure 1.
Relapse-free and overall survival according to treatment with all-trans retinoic acid (ATRA) and genotype on an intention-to-treat basis. (A) Relapse-free survival. (B) Overall survival. Blue, mutant NPM1 without FLT3-ITD and treatment with ATRA; green, mutant NPM1 without FLT3-ITD and no treatment with ATRA; red, all other combinations of the two markers and treatment with ATRA; black, all other combinations of the two markers and no treatment with ATRA.
Table 3.
Cox proportional hazard models for relapse-free and overall survival on an as randomized basis.
Figure 2.
Relapse-free and overall survival according to treatment with all-trans retinoic acid (ATRA) and genotype in patients with cytogenetically normal karyotype on an intention-to-treat basis. (A) relapse-free survival. (B) Overall survival. Blue, mutant NPM1 without FLT3-ITD and treatment with ATRA; green, mutant NPM1 without FLT3-ITD and no treatment with ATRA; red, all other combinations of the two markers and treatment with ATRA; black, all other combinations of the two markers and no treatment with ATRA.
Discussion
In the randomized AML HD98B trial of the AMLSG, we previously showed that ATRA given after intensive chemotherapy significantly improved the outcome of older patients with non-APL AML.12 Data from the current correlative study suggest that this effect was accounted for by patients whose leukemic cells harbor mutant NPM1, and, more specifically, exhibit the genotype ‘mutant NPM1 without FLT3-ITD’. Mutant NPM1, in addition to cytogenetics, emerged as a strong favorable prognostic factor for achievement of complete remission. The updated survival analyses reported here confirm our previous observation that ATRA significantly improved survival in older AML patients.12 Randomized trials from three groups evaluating ATRA as an adjunct to intensive chemotherapy in non-APL AML have been published.8–12 However, in contrast to the positive results of our trial, the results of all other studies had been negative.8–11 The selection of patients, the choice of the chemotherapeutic agents that were used in combination with ATRA, and the schedule of ATRA administration are variables that differed partly between the trials and thus might explain the discrepant results. In our view, one issue deserves special attention. Data from in vitro experiments with AML blasts have suggested that the addition of ATRA to cytotoxic agents, such as cytarabine or idarubicin, increases the killing of clonogenic cells. Importantly, these studies indicated that the schedule of ATRA administration may play a crucial role because synergistic effects on cell viability were only observed when ATRA was administered after exposure to the cytotoxic drug.1–5 Consistent with these observations, Tabe et al. recently showed that pretreatment of the APL cell line NB4 with ATRA upregulates the transmembrane drug transporter ABCB1 (also known as MDR1) and induces doxorubicin resistance.28 In the study by Estey et al.,8 ATRA was started 2 days prior to chemotherapy, and in the MRC trials,9–11 ATRA was started simultaneously with chemotherapy. In contrast, in the AML HD98B trial, ATRA was started on the third day of chemotherapy, a time point when a significant proportion of the cytotoxic drugs had already been administered.
In exploratory analyses, we were able to attribute the beneficial impact of ATRA on relapse-free survival and overall survival to a specific, genetically defined subgroup of patients. Univariable and multivariable analyses pointed to an interaction between ATRA treatment and the genotype ‘mutant NPM1 without FLT3-ITD’, with the beneficial effect of ATRA being restricted to this subgroup of patients. Patients with the genotype ‘mutant NPM1 without FLT3-ITD’ who had been randomized to ATRA had a significantly better outcome compared to patients with the same genotype who had not been randomized to ATRA (Figures 1 and 2).
The exact molecular mechanism through which ATRA may exert its effects in AML with mutant NPM1 remains elusive. However, recent studies have suggested a link between NPM1 and retinoic acid-mediated transcriptional regulation under physiological conditions as well as in myeloid leukemogenesis. First, NPM1 seems to function as a transcriptional co-repressor during retinoic acid-induced cell differentiation.29 Second, the NPM1 gene is involved in a chromosomal translocation, t(5;17)(q35;q21), which is present in a rare variant of APL, and the resulting ATRA-sensitive NPM1-RARA fusion protein has been shown to possess aberrant transcriptional regulatory activity.30–32 Third, Martelli et al.33 showed, in the NPM1 mutant cell line OCI-AML3 and in primary NPM1 mutant leukemias propagated in NOD-SCID mice, that pharmacological doses of ATRA induce cell cycle arrest and apoptosis by selectively downregulating the mutant NPM1 protein.
In summary, our data suggest that mutant NPM1, and more specifically the genotype mutant NPM1 without FLT3-ITD, is a predictive factor for response to ATRA given as an adjunct to intensive chemotherapy in older patients with AML. These findings are currently being validated prospectively in our AMLSG 07-04 treatment protocol in which 920 younger adults 18 to 60 years of age with AML are randomized to intensive combination chemotherapy with or without ATRA. In addition, the results of our study support the concept of systematic molecular genetic studies in AML patients, not only for the evaluation of biomarkers for prognostication, but also for the identification of predictive factors for response to novel therapies.
Acknowledgments
we thank the members of the German-Austrian AMLSG for providing leukemia specimens and clinical data. We are also grateful to Simone Miller and Christa Wieland for technical support.
Appendix
The following co-investigators of the AML HD98B trial contributed between one and nine patients to this study: Axel Glasmacher, University of Bonn; Hans-G. Mergenthaler, Klinikum Stuttgart; Christoph Nerl, Klinikum München-Schwabing; Hans Pralle, University of Giessen; Manfred Hensel, University of Heidelberg; Joachim Preiss, Caritas-Klinik St. Theresia Saarbrücken; Hans Salwender, Klinikum Hamburg-Altona; Hans-G. Biedermann, Kreiskrankenhaus Trostberg; Stephan Kremers, Caritas-Krankenhaus Lebach; Frank Griesinger, University of Göttingen.
Footnotes
Funding: supported in part by grants 01GI9981 [Network of Competence Acute and Chronic Leukemias], 01KG0605 [IPD-meta-analysis: a model-based hierarchical prognostic system for adult patients with acute myeloid leukemia (AML)] from the Bundesministerium für Bildung und Forschung (BMBF), Germany, the Deutsche José Carreras Leukämie-Stiftung (DJCLS R 06/06v), and the Else Kröner-Fresenius-Stiftung (P38/05//A49/05//F03).
Authorship and Disclosures
RFS, KD, SF, HD: designed the research and wrote the paper; AB: reviewed biometrical analyses; DS, SG: analyzed results; LB, MH, BK, SK, AC, AA: performed experiments; MK, KG, FH, FV, HK, EK, JF: co-investigators who contributed more than nine patients, participated in designing the clinical study, and reviewed and approved the paper. The authors reported no potential conflicts of interest.
References
- 1.Hu ZB, Minden MD, McCulloch EA. Direct evidence for the participation of bcl-2 in the regulation by retinoic acid of the Ara-C sensitivity of leukemic stem cells. Leukemia. 1995;9:1667–73. [PubMed] [Google Scholar]
- 2.Yang GS, Minden MD, McCulloch EA. Influence of schedule on regulated sensitivity of AML blasts to cytosine arabinoside. Leukemia. 1993;7:1012–9. [PubMed] [Google Scholar]
- 3.Andreeff M, Jiang S, Zhang X, Konopleva M, Estrov Z, Snell VE, et al. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13:1881–92. doi: 10.1038/sj.leu.2401573. [DOI] [PubMed] [Google Scholar]
- 4.Ketley NJ, Allen PD, Kelsey SM, Newland AC. Modulation of idarubicin-induced apoptosis in human acute myeloid leukemia blasts by all-trans retinoic acid, 1,25(OH)2 vitamin D3, and granulocyte–macrophage colony-stimulating factor. Blood. 1997;90:4578–87. [PubMed] [Google Scholar]
- 5.Hu ZB, Minden MD, McCulloch EA. Phosphorylation of BCL-2 after exposure of human leukemic cells to retinoic acid. Blood. 1998;92:1768–75. [PubMed] [Google Scholar]
- 6.Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784–90. doi: 10.1182/blood.v97.9.2784. [DOI] [PubMed] [Google Scholar]
- 7.Venditti A, Stasi R, Del Poeta G, Buccisano F, Aronica G, Bruno A, et al. All-trans retinoic acid and low-dose cytosine arabinoside for the treatment of poor prognosis acute myeloid leukemia. Leukemia. 1995;9:1121–5. [PubMed] [Google Scholar]
- 8.Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H, et al. Randomized phase II study of fludarabine+cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–84. [PubMed] [Google Scholar]
- 9.Burnett AK, Milligan D, Hills RK, Goldstone AH, Prentice AG, Wheatley K, et al. Does all-trans retinoic acid (ATRA) have a role in non-APL acute myeloid leukaemia¿ Results from 1666 patients in three MRC trials. Blood. 2004;104:1794. [Abstract] [Google Scholar]
- 10.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–24. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 11.Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK NCRI Haematological Oncology Clinical Studies Group. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006;107:4614–22. doi: 10.1182/blood-2005-10-4202. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk RF, Fröhling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F, et al. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–803. doi: 10.1038/sj.leu.2403528. [DOI] [PubMed] [Google Scholar]
- 13.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 14.Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 15.Fröhling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Döhner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–8. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 16.Schlenk RF, Fröhling S, Hartmann F, Fischer JT, Glasmacher A, Del Valle F, et al. Intensive consolidation versus oral maintenance therapy in patients 61 years or older with acute myeloid leukemia in first remission: results of second randomization of the AML HD98-B treatment trial. Leukemia. 2006;20:748–50. doi: 10.1038/sj.leu.2404122. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR. Proposed revised criteria for the classification of acute myeloid leukemia. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 18.Schlenk RF, Hartmann F, Hensel M, Jung W, Weber-Nordt R, Gabler A, et al. Less intense conditioning with fludarabine, cyclophosphamide, idarubicin and etoposide (FCIE) followed by allogeneic unselected peripheral blood stem cell transplantation in elderly patients with leukemia. Leukemia. 2002;16:581–6. doi: 10.1038/sj.leu.2402423. [DOI] [PubMed] [Google Scholar]
- 19.Fröhling S, Kayser S, Mayer C, Miller S, Wieland C, Skelin S, et al. Diagnostic value of fluorescence in situ hybridization for the detection of genomic aberrations in older patients with acute myeloid leukemia. Haematologica. 2005;90:194–9. [PubMed] [Google Scholar]
- 20.Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 21.Fröhling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–33. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 22.Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 23.Döhner K, Tobis K, Ulrich R, Fröhling S, Benner A, Schlenk RF, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–61. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–9. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 25.Korn EL. Censoring distributions as a measure of follow-up in survival analysis. Stat Med. 1986;5:255–60. doi: 10.1002/sim.4780050306. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies: With Applications To Linear Models, Logistic Regression, And Survival Analysis. New York, NY: Springer Verlag; 2001. [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2007. [Google Scholar]
- 28.Tabe Y, Konopleva M, Contractor R, Munsell M, Schober WD, Jin L, et al. Up-regulation of MDR1 and induction of doxorubicin resistance by histone deacetylase inhibitor depsipeptide (FK228) and ATRA in acute promyelocytic leukemia cells. Blood. 2006;107:1546–54. doi: 10.1182/blood-2004-10-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Tan BC, Tseng KH, Chuang CP, Yeh CW, Chen KD, et al. Nucleophosmin acts as a novel AP2alpha-binding transcriptional corepressor during cell differentiation. EMBO Rep. 2007;8:394–400. doi: 10.1038/sj.embor.7400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(15;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996;87:882–6. [PubMed] [Google Scholar]
- 31.Redner RL, Corey SJ, Rush EA. Differentiation of t(5;17) variant acute promyelocytic leukemia blasts by all-trans retinoic acid. Leukemia. 1997;11:1014–6. doi: 10.1038/sj.leu.2400661. [DOI] [PubMed] [Google Scholar]
- 32.Redner RL, Chen JD, Rush EA, Li H, Pollock SL. The t(5;17) acute promyelocytic leukemia fusion protein NPM-RAR interacts with corepressor and co-activator proteins and exhibits both positive and negative transcriptional properties. Blood. 2000;95:2683–90. [PubMed] [Google Scholar]
- 33.Martelli MP, Pettirossi V, Manes N, Liso A, Mezzasoma F, Cecchetti F, et al. Selective silencing of the NPM1 mutant protein and apoptosis induction upon ATRA in vitro treatment of AML cells carrying NPM1 mutations. Blood. 2007;110:868. [Abstract] [Google Scholar]