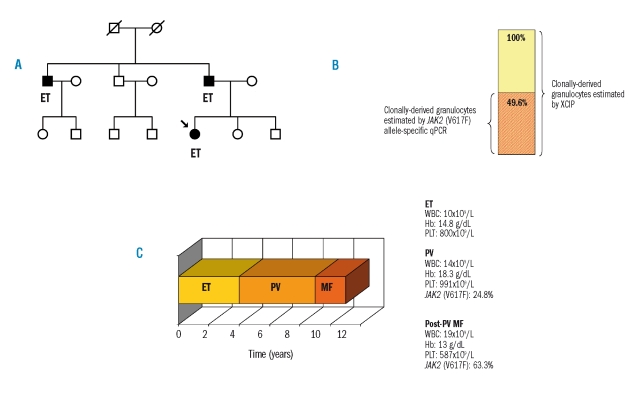
Figure 1.
Genetic predisposition and gene-dosage effect of JAK2 (V617F) in a single patient with familial essential thrombocythemia (ET), who progressed to polycythemia vera (PV) and post-PV myelofibrosis (post-PV MF). (A) Pedigree of the family. The proband (indicated by an arrow) had ET, as did her father and uncle. The familial cluster supports the hypothesis of an inherited genetic predisposition. (B) Comparison of the proportion of clonally derived granulocytes determined by X-chromosome inactivation pattern (XCIP) (yellow bar) and by JAK2 (V617F) allele-specific quantitative polymerase chain reaction (qPCR) (red striped bar) at evolution into PV. The 24.8% mutant allele burden translated into 49.6% of granulocytes being heterozygous for the JAK2 (V617F), or 24.8% of granulocytes being homozygous for the mutation. This supports the existence of a pre-JAK2 phase as a low allele burden exists within a milieu of clonal hematopoiesis. (C) Scheme of the clinical course of the disease over time. Representation of evolution from ET to PV and to post-PV MF. The right side of the panel reports hematologic (white blood cell count, WBC; hemoglobin concentration, Hb: platelet count, PLT) and molecular data. The critical role of a gene dosage effect on the progression of the disease is highlighted.