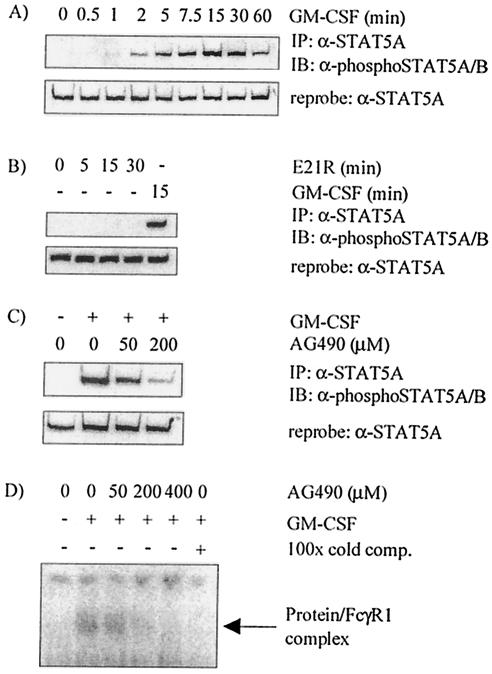
FIG. 1.
STAT5A is phosphorylated on tyrosine 694 in response to GM-CSF stimulation of MDM, and STAT5A activation is inhibited by the JAK2 inhibitor AG490. Monocytes were cultured for 7 days before the following treatment: stimulation with 10 ng of GM-CSF per ml for 0 to 60 min (A); stimulation with 1 μg of E21R (mutant GM-CSF) per ml or with 10 ng of wild-type GM-CSF per ml for the times shown (B); or preincubation with AG490 for 4 h, stimulation with 20 ng of GM-CSF per ml for 5 min, and lysis with RIPA buffer (C). Immunoprecipitation (IP) of equal amounts of cell protein was performed with an anti-STAT5A antibody (α-STAT5A), and blots were probed with anti-phospho-STAT5A/B antibody (α-phosphoSTAT5A/B). Equivalent protein levels are shown by reprobing (after stripping) the blots with anti-STAT5A antibody. Results shown are representative of four independent experiments using MDM isolated from different donors. IB, immunoblotting. (D) Nuclear lysates were generated from MDM preincubated with AG490 for 4 h before stimulation with 10 ng of GM-CSF (+) per ml for 15 min. EMSA was performed using a 32P-labeled oligonucleotide containing the STAT response element derived from the FcγR1 promoter. In each reaction mixture, the same amount of protein was incubated with labeled oligonucleotide, sonicated salmon sperm DNA, and DTT in a solution consisting of 10 mM HEPES, 40 mM NaCl, 1 mM EDTA, and 4% glycerol for 15 min at room temperature. Extract was preincubated (+) with unlabeled competing probe (cold comp.) for 30 min. The gel was dried, and DNA-protein complexes were visualized via autoradiography. Results are representative of MDM isolated from two donors.