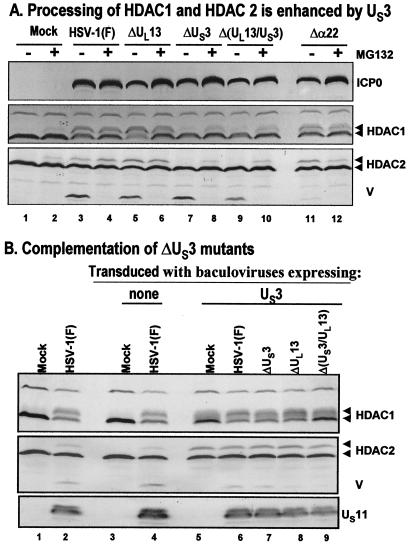
FIG. 3.
Modification of HDAC1 and HDAC2. (A) Processing of HDAC1 and HDAC2 is enhanced by viral protein kinase US3. Replicate cultures of RSC in 25-cm2 flasks were either mock infected or infected with wild-type HSV-1(F) or mutant R7356 (ΔUL13), R7041 (ΔUS3), R7353 (ΔUL13/ΔUS3), or R7802 (Δα22). Cell cultures were infected with 5 PFU of virus per cell. At 3 h after infection, one set of cultures was replenished with fresh untreated medium (− lanes), whereas a second set was replenished with medium containing 10 μM MG132 (+ lanes). The cells were harvested 20 h after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibodies to HDAC1 and HDAC2, and monoclonal antibody to ICP0. The positions of HDAC1 and HDAC2 and of bands reacting with HDAC2 antibody (V) and with monoclonal antibody to ICP0 are indicated to the right of the gel. (B) Complementation of ΔUS3 mutants by baculovirus expressing US3. Replicate RSC cultures in 25-cm2 flasks were transduced with baculoviruses that were either empty of inserts (lanes 3 and 4) or encoding the US3 protein kinase (lanes 5 to 9). At 12 h after transduction, cells were mock infected (lanes 3 and 5) or infected with wild-type HSV-1(F) (lanes 4 and 6) or mutant virus R7356 (ΔUL13), R7041 (ΔUS3), or R7353 (ΔUL13/ΔUS3) (lanes 7 to 9). Cell cultures were infected with 5 PFU of virus per cell. At 12 h after infection with wild-type HSV-1 or mutant viruses, the cultures were harvested and processed as described above except that cell lysates were reacted with anti-US11 antibody rather than ICP0. Lanes 1 and 2 show protein profiles of mock-infected and wild-type virus-infected cell cultures not previously exposed to baculoviruses.