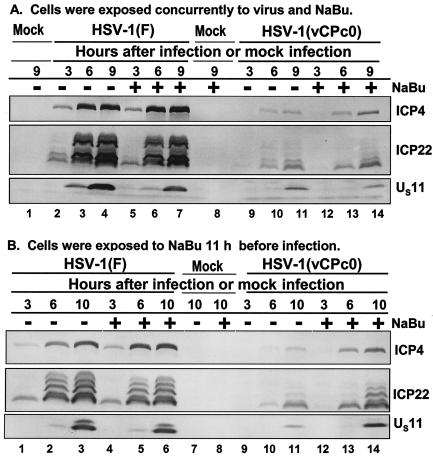
FIG. 4.
Temporal pattern of accumulation of selected wild-type HSV-1(F) and mutant HSV-1(vCPc0) proteins in RSC treated with sodium butyrate (NaBu) (A) at the time of infection and (B) 11 h before infection. (A) Replicate cultures of RSC in 25-cm2 flasks were either mock infected (lanes 1 and 8) or infected with 5 PFU of wild-type HSV-1(F) per cell (lanes 2 to 7) or with 5 PFU of mutant HSV-1(vCPc0) per cell (lanes 9 to 14) in the absence (−) or presence (+) of 6 mM sodium butyrate. The cells were harvested at the indicated times after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibody to ICP22 and monoclonal antibodies to ICP4 and US11. (B) Replicate cultures of RSC in 25-cm2 flasks were either mock treated (lanes 1 to 3, 7, and 9 to 11) or treated (lanes 4 to 6, 8, and 12 to 14) with 6 mM sodium butyrate. At 11 h after treatment, cells were either mock infected (lanes 7 and 8) or infected with 5 PFU of wild-type HSV-1(F) per cell (lanes 1 to 6) or with 5 PFU of mutant HSV-1(vCPc0) per cell (lanes 9 to 14) in the absence (−) or presence (+) of 6 mM sodium butyrate. Cells were harvested at the indicated times after infection. Proteins were solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, and reacted with polyclonal antibody to ICP22 and monoclonal antibodies to ICP4 and US11.