Abstract
The murine gammaherpesvirus 68 (MHV-68 or γHV-68) model provides many advantages for studying virus-host interactions involved in gammaherpesvirus replication, including the role of cellular responses to infection. We examined the effects of cellular cyclooxygenase-2 (COX-2) and its by-product prostaglandin E2 (PGE2) on MHV-68 gene expression and protein production following de novo infection of cultured cells. Western blot analyses revealed an induction of COX-2 protein in MHV-68-infected cells but not in cells infected with UV-irradiated MHV-68. Luciferase reporter assays demonstrated activation of the COX-2 promoter during MHV-68 replication. Two nonsteroidal anti-inflammatory drugs, a COX-2-specific inhibitor (NS-398) and a COX-1-COX-2 inhibitor (indomethacin), substantially reduced MHV-68 protein production in infected cells. Inhibition of viral protein expression and virion production by NS-398 was reversed in the presence of exogenous PGE2. Global gene expression analysis using an MHV-68 DNA array showed that PGE2 increased production of multiple viral gene products, and NS-398 inhibited production of many of the same genes. These studies suggest that COX-2 activity and PGE2 production may play significant roles during MHV-68 de novo infection.
The gamma subfamily of herpesviruses, including Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (HHV-8), are associated with tumors and lymphoproliferative disorders (29, 43). Murine gammaherpesvirus 68 (MHV-68) is phylogenetically related to EBV and KSHV and provides major research advantages by forming plaques on cell monolayers and establishing productive lytic and latent infections in mice (10, 64-66). Unlike EBV and KSHV, MHV-68 readily establishes productive infections in many cell culture systems and thus facilitates the examination of gammaherpesvirus replication and de novo infection (66, 75, 76). This provides an opportunity to examine cellular responses to de novo infection and their role in regulating gammaherpesvirus activity.
Prostaglandins (PGs) are potent immunoregulatory lipid mediators generated by arachidonic acid metabolism via two cellular cyclooxygenases, constitutive COX-1 and inducible COX-2 (21, 22, 26, 61). PGs are formed by most cell types and exert a variety of actions in various tissues and cells via PG receptors (2, 16, 19, 33, 47). PGE2 is an important proinflammatory prostanoid that mediates many symptoms of inflammation (41, 53, 56, 70). Production of PGE2 is catalyzed by COX-2, which is induced in response to factors such as bacterial lipopolysaccharides, mitogens, and cytokines (17, 31, 57, 61). However, it is unknown what role the COX-2-PGE2 pathway might play in responding to gammaherpesvirus de novo infection.
Many viruses have been linked to the modulation of COX-2 expression and PG production (9, 11, 25, 44, 46, 52, 60, 63, 77-79). COX-2 is responsible for the exaggerated biosynthesis of PGs under acute inflammatory conditions and in a diverse group of tumors (14, 23, 32, 67). The COX-1 and COX-2 isozymes are the pharmacologic targets of nonsteroidal anti-inflammatory drugs (NSAIDs) (26, 50, 61, 71), and NSAIDs that block COX activity and PG production have been recognized as potentially effective antiviral therapeutics (4, 6-8, 51, 62, 73, 79). However, the effect of COX inhibitors on de novo infection of a gammaherpesvirus has not been previously examined. MHV-68 has been used as a model to study the efficacy of other antiviral compounds (3, 48, 49, 68), and we used this system to address the effects of COX-2 inhibition on gammaherpesvirus replication and infection.
To enhance our understanding of virus-host cell interactions involved in the replication and pathogenesis of gammaherpesviruses, we examined MHV-68 de novo infection of NIH 3T3 and BHK-21 cells as a model for analyzing the role of PG production and COX-2 activity. We compared the effects of MHV-68 and UV-irradiated MHV-68 infection on COX-2 protein expression and COX-2 promoter activation. COX inhibitors {NS-398 [N-(2-cyclohexyloxy-4-nitrophenyl)-methanesulfonamide] and indomethacin} were tested for their ability to suppress MHV-68 protein expression during de novo infection. Inhibition of protein expression and virion production by NS-398 was relieved in the presence of exogenous PGE2, a product of COX-2 activity. The effects of PGE2 and NS-398 on MHV-68 gene expression were surveyed using MHV-68 DNA arrays. Our results indicate that COX-2 activity and PG production (e.g., PGE2) can significantly influence the transcription program of MHV-68 in de novo infection of cultured cells.
MATERIALS AND METHODS
Virus stocks, cell lines, plaque assays, and reagents.
Viral stocks of wild-type (wt) MHV-68 (ATCC VR1465) and enhanced green fluorescent protein (EGFP)-MHV-68 were prepared as previously described (76). Recombinant EGFP-MHV-68 (tw25) was constructed by insertion of the human cytomegalovirus (HCMV) immediate-early promoter-driven EGFP cassette at the left end of the MHV-68 genome (75). Cell lines were grown at 37°C in the presence of 5% CO2. NIH 3T3 (murine embryonic fibroblasts) and BHK-21 (baby hamster kidney fibroblasts) cells were maintained in Dulbecco's modified Eagle's medium supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), and 10% fetal bovine serum (FBS). As specified, some assays were carried out in low-serum conditions by seeding cells into 0.5% FBS 12 to 18 h before the experiment. Viral titer was determined by plaque assays, using BHK-21 monolayers overlaid with medium containing 1% methylcellulose as previously described (76). UV-irradiated virus was prepared by treating wt MHV-68 stocks with multiple doses of UV irradiation (9 mJ) using a Stratalinker (Stratagene, La Jolla, Calif.) (40). NS-398 (BIOMOL, Plymouth Meeting, Pa.) was reconstituted in dimethyl sulfoxide (DMSO), indomethacin (Calbiochem, La Jolla, Calif.) was reconstituted in ethanol, and PGE2 (Cayman Chemical, Ann Arbor, Mich.) was reconstituted in phosphate-buffered saline.
Western blot analysis.
NIH 3T3 or BHK-21 monolayers were infected with viral inoculum for 1 h, and then the inoculum was replaced with fresh medium. Cells were lysed in 1× passive lysis buffer (Promega, Madison, Wis.), and the total protein concentration of each cell extract was determined using a Bradford assay (Bio-Rad, Hercules, Calif.). Cell extracts were boiled in Laemmli buffer, as described in reference 76, for 5 min and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Broad-range prestained protein standards (Bio-Rad) were included as molecular weight markers. Proteins were electrotransferred (Bio-Rad) to nitrocellulose membranes (Amersham Pharmacia Biotech, Arlington Heights, Ill.) and blocked in phosphate-buffered saline with 0.1% Tween 20 and 5% nonfat milk. Western blots of COX-2 expression were incubated with a COX-2 polyclonal antibody (Cayman Chemical) at a dilution of 1:1,000. Western blots of MHV-68 protein expression were incubated with rabbit hyperimmune serum against MHV-68-infected rabbit cells (65) (anti-MHV-68 serum), rabbit serum for the recombinant MHV-68 open reading frame (ORF) 26 (capsid) protein, or rabbit serum for the recombinant MHV-68 M9-ORF 65 (capsid) protein (75). As a loading control, membranes were incubated with monoclonal antibody to actin (Sigma Chemical, St. Louis, Mo.). Proteins were visualized using a chemiluminescent detection ECL+PLUS system (Amersham Pharmacia Biotech) and a STORM imaging system (Molecular Dynamics, Sunnyvale, Calif.).
Luciferase reporter assays.
Activation of the COX-2 promoter was measured using the Dual Luciferase kit according to the manufacturer's protocol (Promega). NIH 3T3 cells (0.8 × 105 to 1 × 105/well) were seeded in low-serum Dulbecco's modified Eagle's medium (0.5% FBS) in a 24-well tissue culture plate 12 to 18 h before transfection. Low-serum conditions were maintained during the course of the experiment. First, cells were transfected with 0.4 μg of a COX-2 promoter-firefly luciferase construct (74) and 4 ng of pRL-SV40 (Promega) as a transfection efficiency control using Lipofectamine Plus reagent (Gibco). DNA used for transfection was prepared endotoxin-free (Qiagen, Valencia, Calif.). The next day, transfected cells were infected with wt MHV-68 at a multiplicity of infection of 2 PFU/cell (MOI = 2) or mock infected. Cell extracts were harvested at 3, 8, 12, and 24 h postinfection in 1× passive lysis buffer, and luciferase activities were measured using an LMAX luminometer (Molecular Devices, Sunnyvale, Calif.). Firefly luciferase activities (transfected or infected) for each time point were normalized to parallel transfected or mock-infected controls for each time point.
MHV-68 DNA array.
PCR primers were designed to amplify array elements of the 83 regions of the MHV-68 genome representing 73 known and predicted ORFs (38, 72). The individual PCR products were cloned into pCRII (Stratagene), PCR amplified using primer sequences on the vector, isopropanol precipitated, and resuspended in water to a concentration of 100 ng/ml. The array elements were spotted onto Hybond nylon membranes using the Nunc Replication system (Nalge Nunc International, Naperville, Ill.). The spotted DNA was denatured (0.5 M NaOH, 1.5 M NaCl) and neutralized (0.5 M Tris [pH 7.5], 1.5 M NaCl) and then UV cross-linked to the membranes using a UV Stratalinker (Stratagene). The membranes were stored in the dark at room temperature. BHK-21 cells (6 × 105) were seeded in 60-mm-diameter dishes 12 to 18 h before a 15-min pretreatment in medium containing 1 μM PGE2 (see Fig. 6) or 5 μM NS-398 (see Fig. 7). Untreated and treated cells were infected for 1 h with wt MHV-68 at an MOI of 5 (Fig. 6) or 1 (Fig. 7) and then incubated for 8 h before RNA isolation using Tri-Reagent (MRC, Cincinnati, Ohio). Cells were maintained in medium containing 1 μM PGE2 (Fig. 6) or 5 μM NS-398 (Fig. 7) during the infection and postinfection incubations. Total RNA (2 μg) was used in a reverse transcription reaction primed with oligo(dT) primers. [α-32P]dATP-labeled cDNA was prepared using a Strip-EZ kit (Ambion, Austin, Tex.). The arrays were preincubated for 5 h at 65°C before hybridization to the labeled cDNA for 15 h at 65°C. The gene arrays were washed, and the signal intensity was quantitated using a STORM imaging system and ImageQuant software (Molecular Dynamics). Each array element was represented by four individual spots, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) array elements were spotted in each corner of the DNA array. Local background values were subtracted from the signal intensity values for each spot. For each array element, the average of the two median values was normalized to the calculated average GAPDH value for the blot. To calculate fold difference, the normalized value for each array element from treated membranes was divided by the normalized value for each array element from the untreated membranes. Changes in gene expression were quantified by a paired t test on four replicate spots for each ORF in untreated samples versus those treated with PGE2 or NS-398. Linear regression and Pearson's correlation coefficient were used to assess the relationship between the magnitude of a gene's induction by PGE2 and its suppression by NS-398.
FIG. 6.
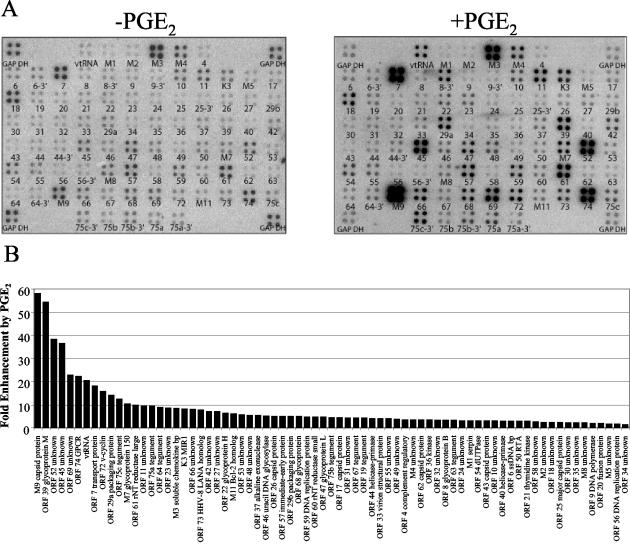
Upregulation of MHV-68 gene expression by PGE2. (A) BHK-21 cells were untreated (left panel) or pretreated with 1 μM PGE2 (right panel) before infection with wt MHV-68. Cells were maintained in medium containing 1 μM PGE2 during the infection and postinfection incubation (right panel). Total RNA was harvested 8 h postinfection, and labeled cDNA probe was generated for hybridization to MHV-68 DNA arrays. The ORFs are printed below each array element. (B) A STORM phosphorimager and ImageQuant system were used to quantitate the signal from the array elements corresponding to 73 known and predicted MHV-68 ORFs. GAPDH-normalized values from the array with PGE2 (A, right panel) were divided by the corresponding GAPDH-normalized values from the untreated array (A, left panel) to derive the fold induction of gene expression relative to the untreated level for each array element. These values and their corresponding MHV-68 ORFs are ordered in the bar graph based on increasing fold induction of gene expression relative to the untreated level. Statistical significance of differences in expression was assessed by paired t test.
FIG. 7.
Downregulation of MHV-68 gene expression by NS-398. (A) BHK-21 cells were untreated (left panel) or pretreated with 5 μM NS-398 (right panel) before infection with wt MHV-68. Cells were maintained in medium containing 5 μM NS-398 during the infection and postinfection incubations (right panel). Total RNA was harvested 8 h postinfection, and labeled cDNA probe was generated for hybridization to MHV-68 DNA arrays. The ORFs are printed below each array element. (B) A STORM phosphorimager and ImageQuant system were used to quantitate the signal from the array elements corresponding to 73 known and predicted MHV-68 ORFs. GAPDH-normalized values from the array with NS-398 (A, right panel) were divided by the corresponding GAPDH-normalized values from the untreated array (A, left panel) to derive the fold inhibition of gene expression relative to the untreated level for each array element. These values and their corresponding MHV-68 ORFs are ordered in the bar graph based on increasing fold inhibition of gene expression relative to the untreated level. Statistical significance of differences in expression was assessed by paired t test.
RESULTS
Induction of COX-2 protein during MHV-68 de novo infection.
COX-2 protein expression during MHV-68 infection was examined by Western blot analysis using a COX-2 polyclonal antibody. COX-2 protein levels were induced during a time course assay of MHV-68 infection of fibroblast cells (Fig. 1A, top panel). NIH 3T3 cells were uninfected (lane 6) or infected with EGFP-MHV-68 (MOI = 2), and cell extracts were harvested at various times (1, 5, 9, and 24 h) following inoculation (lanes 1 to 4). Recombinant EGFP-MHV-68 expresses EGFP and exhibits wt kinetics of replication in tissue culture cells (75). As a positive control for COX-2 induction, NIH 3T3 cells grown in low-serum conditions were treated with a serum shock for 5 h (lane 5).
FIG. 1.
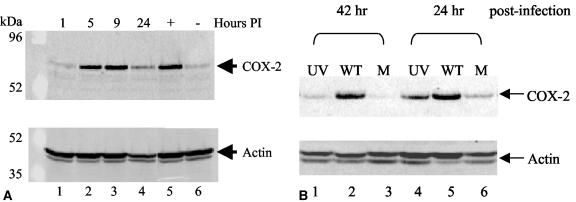
MHV-68 de novo infection increases COX-2 protein levels and requires viral transcription. (A) COX-2 protein expression was induced during MHV-68 replication. NIH 3T3 cells in low-serum conditions were uninfected (lane 6) or infected with EGFP-MHV-68 (lanes 1 to 4). As a positive control, uninfected NIH 3T3 cells were treated with a serum shock (lane 5). Cell extracts were harvested at 1, 5, 9, or 24 h postinfection (PI) and analyzed by Western blotting using a COX-2 polyclonal antibody (top panel). Each lane contained 15 μg of total protein as determined by Bradford assays. The membrane was reprobed with antiactin providing a loading control (bottom panel). (B) COX-2 protein expression was not induced by UV-irradiated MHV-68 compared to wt MHV-68. NIH 3T3 cells in low-serum conditions were infected with wt MHV-68 (lanes 2 and 5), infected with an equal volume of UV-irradiated wt MHV-68 (lanes 1 and 4), or uninfected (lanes 3 and 6). Cell extracts were harvested 24 and 42 h postinfection for Western blot analysis using a COX-2 polyclonal antibody. Each lane contains 15 μg of total protein as determined by Bradford assays. The membrane was reprobed with antiactin, providing a loading control (bottom panel).
It was possible that the virus inoculum contained nonviral elements that induced COX-2 protein expression. To control for this and to determine if viral transcription was required for COX-2 induction during MHV-68 infection, additional experiments were performed. Infection with UV-irradiated wt MHV-68 induced COX-2 protein expression to levels just above background levels, as determined by Western blot analysis using a COX-2 polyclonal antibody (Fig. 1B, top panel). wt MHV-68 virions were inactivated by UV irradiation that causes DNA damage and prevents viral gene transcription. The UV-irradiated virus titer was 1.3 × 105 times lower than the wt MHV-68 titer. NIH 3T3 cells in low-serum conditions were infected with wt MHV-68 (MOI = 2) (WT, lanes 2 and 5), infected with an equal volume of UV-irradiated MHV-68 (UV, lanes 1 and 4), or mock infected (M, lanes 3 and 6), and cell extracts were harvested at 24 and 42 h postinfection. Infection of cells with wt MHV-68 induced COX-2 protein; however, cells infected with UV-irradiated MHV-68 exhibited levels of COX-2 protein slightly above the mock-infected controls. Similar results were obtained at 8 and 32 h postinfection (data not shown). In the four time points tested, the induction of cellular COX-2 protein levels by UV-irradiated MHV-68 infection was reduced to near-background levels, in contrast to the prominent induction of cellular COX-2 during wt MHV-68 infection performed in parallel. These results suggest that MHV-68 infection induces COX-2 protein via mechanisms that depend on viral gene expression.
Induction of COX-2 promoter activity during MHV-68 de novo infection.
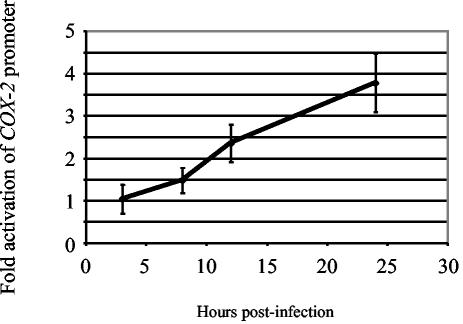
Luciferase reporter assays were used to analyze COX-2 promoter activation during MHV-68 infection. Transcription from the COX-2 promoter was activated during a time course assay of MHV-68 infection of transiently transfected cells (Fig. 2). NIH 3T3 cells were cotransfected with a COX-2 promoter-luciferase construct (74) and a Renilla luciferase plasmid control. Transfected cells were subsequently infected with wt MHV-68 (MOI = 2), and cell extracts were harvested at various times postinfection (3, 8, 12, and 24 h) for analysis. Luciferase reporter assays consistently demonstrated increasing activation of the COX-2 promoter up to fourfold above background control levels at 24 h postinfection. As a positive control, the same experiment was performed simultaneously with a MHV-68 ORF 57-luciferase construct in place of the COX-2 promoter construct. The ORF 57 promoter is known to be highly upregulated during MHV-68 replication (36, 75), and the ORF 57 promoter showed strong induction following MHV-68 infection of NIH 3T3 cells (data not shown).
FIG. 2.
MHV-68 replication activates the COX-2 promoter. NIH 3T3 cells were transfected with a COX-2 promoter-luciferase construct (74) and a pRL-SV40 control plasmid. Transfected cells were infected with wt MHV-68 or mock infected. Cell extracts were harvested at 3, 8, 12, and 24 h postinfection, and luciferase activities were measured and normalized to control samples. Each data point represents four separate experiments performed in duplicate.
Inhibition of MHV-68 protein expression in NIH 3T3 cells by NS-398 and indomethacin.
The induction of COX-2 protein expression (Fig. 1) and COX-2 promoter activity (Fig. 2) suggests that COX-2 enzyme activity may play a role during MHV-68 de novo infection. Western blot analyses were performed to examine viral protein expression in the presence of the COX-inhibiting NSAIDs NS-398 and indomethacin. NS-398 is a preferential COX-2 inhibitor, while indomethacin is a potent inhibitor of both COX-1 and COX-2 (35, 50). MHV-68 protein expression during infection of NIH 3T3 cells was inhibited by NS-398 and by indomethacin (Fig. 3). NIH 3T3 cells were untreated (lanes 1 and 2) or pretreated with DMSO (lane 3) or 2 (lane 4), 10 (lane 5) or 50 (lane 6) μM NS-398. Similarly, NIH 3T3 cells were pretreated with ethanol (lane 7) or 0.2 (lane 8), 1 (lane 9) or 5 (lane 10) μM indomethacin. Cells were infected with wt MHV-68 (MOI = 5) (lanes 2 to 10) and maintained in the appropriate diluent-drug concentrations until cell extracts were harvested 8 h postinfection for Western blot analyses using anti-MHV-68 serum (top panel) recognizing multiple viral lytic antigens (65) or an antibody to MHV-68 ORF 26 (center panel). Control cells were uninfected (lane 1) or uninfected and treated with NS-398 (lane 11) or indomethacin (lane 12). NS-398 and indomethacin inhibited viral protein expression at concentrations that did not affect cell viability or proliferation as determined by trypan blue exclusion assays (data not shown). Nontoxic concentrations of NS-398 (COX-2 inhibitor) and indomethacin (COX-1 and COX-2 inhibitor) inhibited MHV-68 protein expression in infected cells compared to the no-drug (lane 2) and diluent-only controls (lanes 3 and 7).
FIG. 3.
NS-398 and indomethacin suppress MHV-68 protein expression. NIH 3T3 cells were untreated (lanes 1 and 2) or pretreated with DMSO (lane 3) or 2 (lane 4), 10 (lane 5), or 50 (lane 6) μM NS-398. Similarly, NIH 3T3 cells were pretreated with ethanol (EtOH) only (lane 7) or 0.2 (lane 8), 1 (lane 9), or 5 (lane 10) μM indomethacin. Subsequently, cells were infected with wt MHV-68 (lanes 2 to 10) and maintained in the appropriate diluent-drug concentrations. Cell extracts were harvested 8 h postinfection for Western blot analyses using anti-MHV-68 serum (top panel) or an antibody to MHV-68 ORF 26 (center panel). Control cells were uninfected (lane 1) or uninfected and treated with NS-398 (lane 11) or indomethacin (lane 12). As a loading control, the blot was probed with antiactin (bottom panel). Each lane contains 30 μg of total protein as determined by Bradford assays.
PGE2 relieves NS-398 inhibition of MHV-68 protein expression.
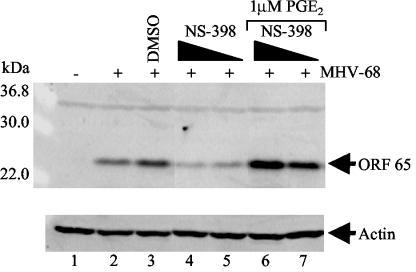
To determine whether reduced PGE2 production mediates the effects of COX-2 inhibitors on MHV-68 protein expression, we sought to reverse the effects of NS-398 by supplementing cultures with exogenous PGE2 (Fig. 4). NIH 3T3 cells were untreated (lanes 1 and 2) or pretreated with DMSO (lane 3) or 5 (lanes 4 and 6) or 0.5 (lanes 5 and 7) μM NS-398 for 30 min. Cells were then infected with wt MHV-68 (MOI = 2) (lanes 2 to 7) and maintained in the appropriate DMSO-NS-398 concentrations. Subsequently, infected cells treated with NS-398 were also treated with 1 μM PGE2 (lanes 6 and 7), and cell extracts were harvested at 12 h postinfection. Control cells were uninfected (lane 1) or infected with no treatment (lane 2). Western blot analysis using an antibody to MHV-68 M9-ORF 65 demonstrated that inhibition of M9-ORF 65 expression by NS-398 was fully relieved in the presence of 1 (lanes 6 and 7) or 0.1 (data not shown) μM exogenous PGE2. Thus, NSAID-induced changes in the COX-2 product PGE2 are sufficient to account for the effect of NS-398 on MHV-68 protein expression.
FIG. 4.
NS-398 suppression of MHV-68 viral protein expression is relieved in the presence of exogenous PGE2. NIH 3T3 cells were untreated (lanes 1 and 2) or pretreated with DMSO (lane 3) or 5 (lanes 4 and 6) or 0.5 (lanes 5 and 7) μM NS-398. Cells were infected with wt MHV-68 (lanes 2 to 7) and maintained in the appropriate DMSO-NS-398 concentrations. Subsequently, infected cells treated with NS-398 were also treated with 1 μM PGE2 (lanes 6 and 7), and cell extracts were harvested at 12 h postinfection for Western blot analysis using an antibody to a late lytic gene product, MHV-68 M9-ORF 65 (upper panel). Control cells were uninfected (lane 1) or infected with no treatment (lane 2). As a loading control, the blot was probed with antiactin (lower panel). Each lane contains 15 μg of total protein as determined by Bradford assays.
Dose-dependent upregulation of MHV-68 protein expression by PGE2.
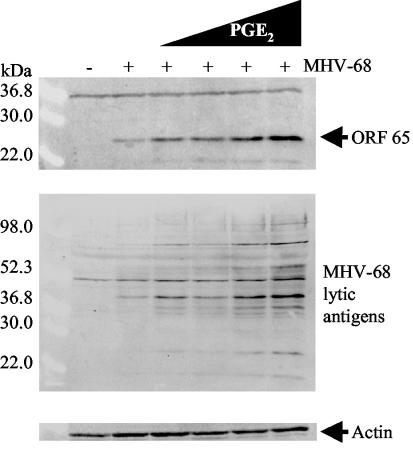
To determine whether PGE2 is a general activator of MHV-68 protein expression in de novo-infected cells or affects only cells subject to COX-2 inhibition, we examined the effect of exogenous PG using Western blot analyses. PGE2 treatment resulted in a dose-dependent enhancement of MHV-68 protein expression (Fig. 5). NIH 3T3 cells were uninfected (lane 1) or infected with wt MHV-68 (MOI = 2) (lanes 2 to 6). Immediately following inoculation, cells were treated with media containing increasing concentrations of PGE2: 0.05 (lane 3), 0.25 (lane 4), 1.25 (lane 5), and 6.25 (lane 6) μM. Control cells were infected and untreated (lane 2). Cell extracts were harvested 12 h postinfection, and protein expression was assayed using an antibody to MHV-68 M9-ORF 65 (top panel). The blot was stripped and reprobed with anti-MHV-68 serum (center panel) that recognizes multiple viral lytic antigens. These studies revealed dose-dependent enhancement of M9-ORF 65 and MHV-68 protein expression in the presence of increasing concentrations of exogenous PGE2.
FIG. 5.
PGE2 exhibits dose-dependent enhancement of MHV-68 viral protein expression. NIH 3T3 cells were uninfected (lane 1) or infected for 1 h with wt MHV-68 (lanes 2 to 6). Immediately following inoculation, cells were treated with increasing concentrations of PGE2: 0.05 (lane 3), 0.25 (lane 4), 1.25 (lane 5), and 6.25 (lane 6) μM. Control cells were infected and untreated (lane 2). Cell extracts were harvested 12 h postinfection, and protein expression was assayed by Western blot analysis using an antibody to MHV-68 M9-ORF 65 (top panel). The blot was stripped and reprobed with anti-MHV-68 serum (center panel). As a loading control, the blot was probed with antiactin (bottom panel). Each lane contains 30 μg of total protein as determined by Bradford assays.
DNA array analysis: PGE2 enhances MHV-68 transcription in BHK-21 cells.
MHV-68 DNA array analysis was used to examine the global effect of PGE2 on MHV-68 gene expression (Fig. 6). BHK-21 cells were infected with wt MHV-68 in the absence or presence of 1 μM PGE2 (Fig. 6A). Total RNA was harvested 8 h postinfection, and labeled cDNA probe was generated and hybridized to MHV-68 DNA arrays. Infected cells treated with PGE2 (right panel) exhibited a marked enhancement of MHV-68 gene expression compared to infected untreated cells (left panel).
A STORM phosphorimager and ImageQuant software were used to quantify signals from the array elements corresponding to 73 known and predicted MHV-68 ORFs (38, 72). GAPDH-normalized values from the array with PGE2 were divided by the corresponding GAPDH-normalized values from the untreated array to derive the fold induction of gene expression relative to the untreated level for each array element. These values and their corresponding viral ORFs are represented in a bar graph ordered by increasing fold induction (Fig. 6B). Gene induction values ranged from 1.4-fold (ORF 34) to 58-fold (M9-ORF 65). Strikingly, none of the 73 known and predicted ORFs on the MHV-68 DNA array was downregulated in response to PGE2.
PGE2 elicited the most profound effect (>10-fold induction) on a subset of 13 MHV-68 genes: M9-ORF 65 (capsid), ORF 39 (glycoprotein M), ORF 52, ORF 45, ORF 69, ORF 74 (GPCR), vtRNA, ORF 7 (transport protein), ORF 72 (v-cyclin), ORF 29a (packaging protein), ORF 75c (tegument), M7 (glycoprotein 150), and ORF 61 (ribonucleotide reductase, large).
DNA array analysis: NS-398 inhibits MHV-68 transcription in BHK-21 cells.
We also used the MHV-68 DNA array to examine the global effect of the COX-2 inhibitor NS-398 on MHV-68 gene expression (Fig. 7). BHK-21 cells were infected with wt MHV-68 in the absence or presence of 5 μM NS-398 (Fig. 7A). Total RNA was harvested 8 h postinfection, and labeled cDNA probe was generated and hybridized to MHV-68 DNA arrays. Infected cells treated with NS-398 (right panel) showed a marked inhibition of viral gene expression compared to infected untreated cells (left panel).
GAPDH-normalized signals from array elements corresponding to each of the 73 known and predicted MHV-68 ORFs (38, 72) were analyzed as above to quantify fold change as a function of NS-398 treatment. These analyses revealed consistent downregulation of MHV-68 genes, with fold of inhibition values ranging from 5.6 (M3) to 1.3 (ORF 29b). These values and their corresponding viral ORFs are represented in a bar graph ordered by increasing fold inhibition of gene expression relative to the untreated control (Fig. 7B). None of the 73 known and predicted ORFs on the MHV-68 DNA array displayed upregulation of gene expression in the presence of NS-398.
NS-398 elicited the most profound inhibitory effect (2.8- to 5.6-fold inhibition) on a subset of 13 MHV-68 genes: M3 (soluble chemokine binding protein), M9-ORF 65 (capsid), ORF 52, ORF 45, ORF 74 (GPCR), ORF 69, ORF 7 (transport protein), ORF 18b, ORF 39 (glycoprotein M), ORF 61 (ribonucleotide reductase, large), ORF 75c (tegument), M7 (glycoprotein 150), and ORF 19 (tegument). Consistent with the Western blot data in Fig. 3 and 4, global expression analysis revealed a suppression of MHV-68 gene transcription by NS-398.
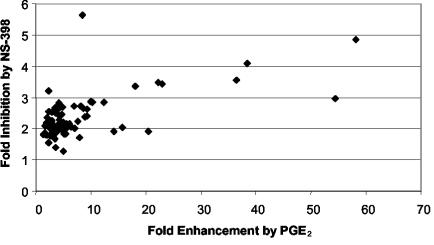
A comparison of the data from Fig. 6 and 7 was made (Fig. 8). Figure 8 compares the magnitude of change in each viral gene product's concentration across these two distinct manipulations of PGE2 signaling, exogenous PGE2 treatment and NS-398 treatment. This analysis revealed that viral genes that were highly induced by exogenous PGE2 also tended to be those showing the greatest magnitude of suppression following COX-2 inhibition by NS-398 treatment. The correlation value of r = 0.6 shows that there is a statistically significant tendency for genes that are heavily induced by exogenous PGE2 to be suppressed by NS-398 treatment.
FIG. 8.
Relationship between MHV-68 gene induction in response to PGE2 and suppression in response to NS-398. Change in expression of each ORF was quantified as described above (Fig. 6 and 7), and magnitude of gene induction in response to PGE2 (horizontal axis) was compared with magnitude of gene suppression in response to NS-398 (vertical axis). Each point represents 1 of 73 assayed MHV-68 ORFs, and the best-fit line was produced by linear regression. The strength of relationship between a gene's induction by PGE2 and its suppression by NS-398 was quantified by Pearson correlation coefficient (r).
PGE2 relieves NS-398 inhibition of MHV-68 virion production.
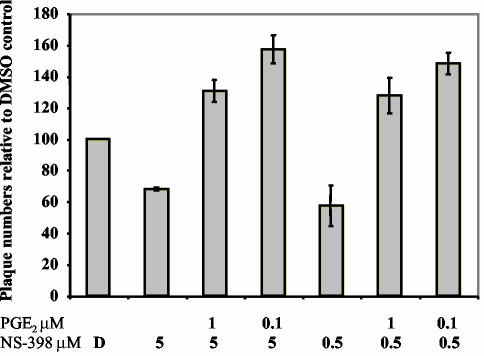
To determine whether reduced PGE2 production mediates the effects of COX-2 inhibitors on MHV-68 infectious virion production, we sought to reverse the effects of NS-398 by supplementing infected cultures with exogenous PGE2 (Fig. 9). NIH 3T3 cells were pretreated for 30 to 45 min with DMSO (D) or NS-398 (0.5 or 5 μM) before infection with wt MHV-68 (MOI = 2) and were maintained in the appropriate DMSO or NS-398 concentrations. Subsequently, some NS-398-treated cells were treated with PGE2 (0.1 or 1 μM). Supernatants were harvested at 12 h postinfection, and viral titer was quantitated using plaque assays on BHK-21 monolayers. The total number of plaques is shown relative to the DMSO control (Fig. 9). The plaque assay results demonstrated that inhibition of virion production (∼35 to 42% inhibition) by NS-398 was fully relieved in the presence of 0.1 or 1 μM PGE2. Cells treated with both NS-398 and PGE2 produced more virions than DMSO-only treated controls. These data suggest that the NSAID-induced changes in the levels of the COX-2 product PGE2 are sufficient to account for the effect of NS-398 on MHV-68 virion production.
FIG. 9.
Inhibition of MHV-68 virion production by NS-398 is reversed by PGE2. NIH 3T3 cells were pretreated with DMSO (D) or NS-398 (0.5 or 5 μM). Subsequently, cells were infected with wt MHV-68 and maintained in the appropriate DMSO-NS-398 concentrations. Immediately after infection, NS-398-treated cells were also treated with PGE2 (0.1 or 1 μM). Supernatants were harvested at 12 h postinfection, and viral titer was quantitated using plaque assays on BHK-21 monolayers.
DISCUSSION
We used the MHV-68 model system to investigate the role of PG production and COX-2 activity during de novo infection by a gammaherpesvirus. Western blot analyses and luciferase reporter assays demonstrated the induction of COX-2 protein and activation of the COX-2 promoter during MHV-68 infection. UV-irradiated MHV-68 infection did not greatly induce COX-2 protein levels compared to wt MHV-68 infection, suggesting that viral transcription is required for this effect. In addition to COX-2 induction by MHV-68, activity of the COX-2 enzyme appears to play a role in supporting viral gene expression. Production of a subset of MHV-68 proteins was suppressed by treatment with a COX-2-specific inhibitor (NS-398) or a COX-1-COX-2 inhibitor (indomethacin). Inhibition of viral protein expression and infectious virion production by NS-398 was relieved by addition of the COX-2 product PGE2. Exogenous PGE2 enhanced MHV-68 protein production and gene transcription even in the absence of COX-2 inhibitors.
Global viral transcription profiling using MHV-68 DNA arrays was performed during two distinct manipulations of PGE2 signaling, treatment with NS-398 and treatment with PGE2. Analyses using NS-398-treated infected cells showed a consistent decrease in MHV-68 gene transcription. Analyses using PGE2-treated infected cells showed a consistent increase in MHV-68 gene transcription. However, all viral genes were not uniformly induced to high levels. The most robust enhancement of viral gene expression by PGE2 was seen for a subset of 13 MHV-68 genes showing >10-fold induction, while other viral genes showed inductions as low as 1.4-fold. In this report, we demonstrate that PGE2 induced protein production and gene expression. We have also examined the effect of exogenous PGE2 on virus production in infected NIH 3T3 cells. These studies revealed that increasing amounts of exogenously added PGE2 did not significantly increase infectious virion production (data not shown). One possible explanation is that an increase in the abundance of a subset of viral gene products may not lead to upregulation of the assembly-export of infectious virions from a cell.
We compared the results of the MHV-68 DNA arrays presented here and found a statistically significant tendency for the genes that showed the greatest induction by PGE2 to also show the greatest repression following COX-2 inhibition by NS-398 treatment (Fig. 8). As seen from the figure axes, the inhibitor has less powerful effects (∼5-fold suppression compared to the 100-fold inductions for the PGE2 treatment), possibly because the basal level of PGE2 produced by infected cells in culture is much lower than the pharmacologic levels achieved by exogenous addition of PGE2. The correlation value of r = 0.6 likely reflects differences in the mechanism of action and intensity of effect between the two treatments. If the two treatments involved totally different mechanisms of action, then we would expect no correlation between their effects on specific genes. We present the data in Fig. 8 to make the point that these two different manipulations of the PGE2 pathway do show more correspondence than we would expect by chance.
Here we demonstrate that NS-398 treatment suppressed MHV-68 gene expression and, to some extent, virion production. Inhibition of infectious virion production by NS-398 was not dramatic (Fig. 9); however, inhibition was fully relieved and viral titers were higher than controls when exogenous PGE2 was present. These results support our data which suggest that a subset of viral genes, but not all, are greatly affected by the PG pathway. These cultured cell experiments are consistent with initial in vivo experiments (data not shown) in which we saw no significant difference in MHV-68 titers in the lungs of intranasally infected mice that were simultaneously treated with or without NS-398.
Several viruses and specific viral gene products are known to upregulate cellular COX-2 expression and activity, including HCMV and its immediate-early proteins (62, 78, 79), hepatitis B virus and the hepatitis B virus X protein (9, 34), human T-cell leukemia virus type 1 (HTLV-1) and the HTLV-1 Tax protein (44, 45), the EBV latent oncoprotein latent membrane protein 1 (LMP1) (46), and HHV-6 and its immediate-early protein 2 (25). Although COX-2-mediated biosynthesis of PGE2 plays a critical role in inflammatory responses to viral infection, it may also be beneficial for certain viruses (42). In the case of MHV-68, viral induction of COX-2 activity appears to significantly enhance the efficiency of viral gene expression following de novo infection. This signaling loop may represent part of the mechanism that the virus utilizes for efficient lytic replication.
The mechanism by which MHV-68 increases COX-2 expression is unknown. For the betaherpesvirus HCMV, viral transcription is not required for the upregulation of COX-2 mRNA levels. A component of the virion particle is thus likely to drive COX-2 upregulation during de novo infection of fibroblast cells (79). In contrast, MHV-68 appears to utilize a different mechanism for COX-2 upregulation during viral replication because viral transcription is required. It is currently unknown if a latent MHV-68 infection modulates COX-2 expression. In the related gammaherpesvirus EBV, LMP-1 promotes COX-2 overexpression in LMP-1-positive nasopharyngeal carcinoma cells during latency (46).
Two COX inhibitors, NS-398 and indomethacin, substantially reduced the production of virally encoded proteins during MHV-68 infection of NIH 3T3 cells. NS-398 inhibits COX-2 enzyme activity in vitro and in vivo, and indomethacin has been used clinically as an inhibitor of both COX-1 and COX-2 (20, 27, 35, 55, 71). Studies of viruses including HCMV, herpes simplex virus types 1 (HSV-1) and HSV-2, vesicular stomatitis virus (VSV), and Japanese encephalitis virus have suggested that NSAIDs that block COX activity and PG production may be effective antiviral agents (4, 6, 8, 28, 62, 73, 79). The data presented here suggest that gammaherpesvirus replication is also sensitive to drugs that block COX-2. Viral gene and protein expression were inhibited using concentrations of NS-398 and indomethacin that did not affect cell viability or proliferation (data not shown). Cell viability is further supported by the results demonstrating that the effects of NS-398 were reversed by exogenous PGE2 and led to the production of viral proteins.
COX-2-mediated production of PGE2 (5, 39) appears to be responsible for the effects of COX-2 inhibitors on MHV-68 protein production (Fig. 4). Exogenous PGE2 enhanced MHV-68 gene and protein expression in the absence of COX-2 inhibitors, and it completely restored viral protein production in the presence of NS-398. These effects are even more pronounced than those seen in experiments showing that PGE2 can partially restore HCMV replication in the presence of a COX inhibitor (79).
Several viruses are sensitive to the antiviral activity of certain PGs, such as the A type (59). Similarly, the proinflammatory E-type PG (PGE2) was previously reported to decrease replication of adenoviruses and parainfluenza virus in vitro (37, 51) and hepatitis B virus in vivo (18, 24), as well as to inhibit CCR5 expression and human immunodeficiency virus type 1 (HIV-1) infection (69). In contrast, PGE2 treatment increased the viral yield of HSV-1, an alphaherpesvirus (28); induced replication of HTLV-1 in infected peripheral blood mononuclear cells (45); and increased levels of bovine leukemia virus tax and pol transcripts (54). Recently, PGE2 treatment was shown to increase viral replication of the betaherpesvirus HHV-6 in peripheral blood mononuclear cells (25). The data presented here demonstrate PGE2 dose-dependent upregulation of viral protein expression and global induction of viral transcription during MHV-68 infection. PGE2 has been shown to enhance activity of at least one member of each herpesvirus subfamily (alphaherpesviruses, betaherpesviruses, and gammaherpesviruses) (58), suggesting that there may be a conserved role for PGE2 during herpesvirus evolution.
The mechanism by which PGE2 modulates virus replication is being examined. PGE2 has been shown to activate several viral promoters, including the HCMV major immediate-early promoter (30), the HTLV-1 long terminal repeat (LTR) promoter (45), and the HIV-1 LTR promoter (12, 13). Western blots and DNA array analysis revealed an increase in M9-ORF 65 protein levels and enhanced transcription of multiple viral genes following exposure to PGE2. PGE2 enhanced activity of MHV-68 in both NIH 3T3 cells (Fig. 5) and BHK-21 cells (Fig. 6), suggesting that its effects are not cell type specific.
DNA array analysis provides an efficient method for examining global changes in MHV-68 gene expression (1, 15, 38). DNA array analyses showed that PGE2 upregulates MHV-68 gene expression, with genes showing inductions ranging up to 58-fold. Genes that were most strongly induced by PGE2 treatment tended to be highly expressed genes, e.g., M9-ORF 65 encoding a capsid protein (58-fold activation) and glycoprotein genes. The viral genes responding the least to PGE2 treatment tended to be less abundantly expressed genes, such as those involved in DNA replication and nucleotide metabolism. MHV-68 DNA array data also demonstrated that COX-2 inhibition produces a global inhibitory effect on MHV-68 gene expression, and again, the most affected genes tended to be highly expressed genes. A comparison was made between the subset of MHV-68 genes most enhanced by PGE2 and the subset of MHV-68 genes most inhibited by NS-398. Strikingly, 10 of the 13 genes overlap: M9-ORF 65, ORF 39, ORF 52, ORF 45, ORF 69, ORF 74, ORF 7, ORF 75c, M7, and ORF 61. Correspondence between PGE2-induced and NS-398-suppressed genes suggests that this core set of MHV-68 genes may be regulated by viral promoters that are particularly sensitive to PG-mediated signal transduction pathways. The gene-regulatory pathway mediating the effects of PGE2 on MHV-68 remains to be defined.
The present data show that de novo infection with MHV-68 induces the expression of COX-2 and that COX-2-mediated production of PGE2 in turn supports MHV-68 gene expression. In addition to identifying one cellular signaling pathway that may be coopted by MHV-68 to support its replication, these data also identify a potential target for antiviral therapy of gammaherpesviruses using COX-2-inhibiting drugs. Blocking key interaction pathways in the virus-host relationship using widely used NSAIDs may help suppress gammaherpesvirus replication and infection, either alone or in combination with other antiherpesvirus therapies.
Acknowledgments
We thank Steve Dubinett for critical discussions.
This work was supported by NIH grants CA83525, CA91791, and DE14153 and the Stop Cancer Foundation (R.S.); the UCLA Asthma, Allergy and Immunologic Disease Center funded by AI-AI50495 from the NIAID and the NIEHS (H.H.); and AI49135, AI52737, and the James L. Pendelton Foundation (S.C.). T.L.S. was supported by a Giannini Foundation-Bank of America Postdoctoral Fellowship and by NIH grant F32 CA88517.
REFERENCES
- 1.Ahn, J. W., K. L. Powell, P. Kellam, and D. G. Alber. 2002. Gammaherpesvirus lytic gene expression as characterized by DNA array. J. Virol. 76:6244-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaraz, M. J., A. Habib, M. Lebret, C. Creminon, S. Levy-Toledano, and J. Maclouf. 2000. Enhanced expression of haem oxygenase-1 by nitric oxide and antiinflammatory drugs in NIH 3T3 fibroblasts. Br. J. Pharmacol. 130:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes, A., H. Dyson, N. P. Sunil-Chandra, P. Collins, and A. A. Nash. 1999. 2′-Deoxy-5-ethyl-beta-4′-thiouridine inhibits replication of murine gammaherpesvirus and delays the onset of virus latency. Antivir. Chem. Chemother. 10:321-326. [DOI] [PubMed] [Google Scholar]
- 4.Bratcher, D. F., C. J. Harrison, N. Bourne, L. R. Stanberry, and D. I. Bernstein. 1993. Effect of indomethacin on ultraviolet radiation-induced recurrent herpes simplex virus disease in guinea-pigs. J. Gen. Virol. 74:1951-1954. [DOI] [PubMed] [Google Scholar]
- 5.Brock, T. G., R. W. McNish, and M. Peters-Golden. 1999. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 274:11660-11666. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. J., S. L. Raung, M. D. Kuo, and Y. M. Wang. 2002. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J. Gen. Virol. 83:1897-1905. [DOI] [PubMed] [Google Scholar]
- 7.Chen, N., A. Restivo, and C. S. Reiss. 2002. Selective inhibition of COX-2 is beneficial to mice infected intranasally with VSV. Prostaglandins Other Lipid Mediat. 67:143-155. [DOI] [PubMed] [Google Scholar]
- 8.Chen, N., J. L. Warner, and C. S. Reiss. 2000. NSAID treatment suppresses VSV propagation in mouse CNS. Virology 276:44-51. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, A. S., H. L. Chan, N. W. Leung, C. T. Liew, K. F. To, P. B. Lai, and J. J. Sung. 2002. Expression of cyclooxygenase-2 in chronic hepatitis B and the effects of anti-viral therapy. Aliment. Pharmacol. Ther. 16:251-260. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, J. P., and P. C. Doherty. 1999. Quantitative analysis of the acute and long-term CD4+ T-cell response to a persistent gammaherpesvirus. J. Virol. 73:4279-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corasaniti, M. T., C. Bellizzi, R. Russo, C. Colica, D. Amantea, and G. Di Renzo. 2003. Caspase-1 inhibitors abolish deleterious enhancement of COX-2 expression induced by HIV-1 gp120 in human neuroblastoma cells. Toxicol. Lett. 139:213-219. [DOI] [PubMed] [Google Scholar]
- 12.Dumais, N., B. Barbeau, M. Olivier, and M. J. Tremblay. 1998. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-κB-dependent and -independent signaling pathways. J. Biol. Chem. 273:27306-27314. [DOI] [PubMed] [Google Scholar]
- 13.Dumais, N., S. Bounou, M. Olivier, and M. J. Tremblay. 2002. Prostaglandin E2-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/enhancer binding protein (C/EBP) binding sites in addition to cooperative interactions between C/EBPβ and cyclic adenosine 5′-monophosphate response element binding protein. J. Immunol. 168:274-282. [DOI] [PubMed] [Google Scholar]
- 14.Eberhart, C. E., R. J. Coffey, A. Radhika, F. M. Giardiello, S. Ferrenbach, and R. N. DuBois. 1994. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183-1188. [DOI] [PubMed] [Google Scholar]
- 15.Ebrahimi, B., B. M. Dutia, K. L. Roberts, J. J. Garcia-Ramirez, P. Dickinson, J. P. Stewart, P. Ghazal, D. J. Roy, and A. A. Nash. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 84:99-109. [DOI] [PubMed] [Google Scholar]
- 16.Elias, J. A., K. Gustilo, W. Baeder, and B. Freundlich. 1987. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J. Immunol. 138:3812-3816. [PubMed] [Google Scholar]
- 17.Evett, G. E., W. Xie, J. G. Chipman, D. L. Robertson, and D. L. Simmons. 1993. Prostaglandin G/H synthase isoenzyme 2 expression in fibroblasts: regulation by dexamethasone, mitogens, and oncogenes. Arch. Biochem. Biophys. 306:169-177. [DOI] [PubMed] [Google Scholar]
- 18.Flowers, M., A. Sherker, S. B. Sinclair, P. D. Greig, R. Cameron, M. J. Phillips, L. Blendis, S. W. Chung, and G. A. Levy. 1994. Prostaglandin E in the treatment of recurrent hepatitis B infection after orthotopic liver transplantation. Transplantation 58:183-192. [PubMed] [Google Scholar]
- 19.Funk, C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871-1875. [DOI] [PubMed] [Google Scholar]
- 20.Futaki, N., S. Takahashi, M. Yokoyama, I. Arai, S. Higuchi, and S. Otomo. 1994. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins 47:55-59. [DOI] [PubMed] [Google Scholar]
- 21.Herschman, H. R. 1996. Prostaglandin synthase 2. Biochim. Biophys. Acta 1299:125-140. [DOI] [PubMed] [Google Scholar]
- 22.Herschman, H. R., R. S. Gilbert, W. Xie, S. Luner, and S. T. Reddy. 1995. The regulation and role of TIS10 prostaglandin synthase-2. Adv. Prostaglandin Thromboxane Leukot. Res. 23:23-28. [PubMed] [Google Scholar]
- 23.Huang, M., M. Stolina, S. Sharma, J. T. Mao, L. Zhu, P. W. Miller, J. Wollman, H. Herschman, and S. M. Dubinett. 1998. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 58:1208-1216. [PubMed] [Google Scholar]
- 24.Hyman, A., C. Yim, M. Krajden, S. Read, A. S. Basinski, I. Wanless, G. Levy, and J. Heathcote. 1999. Oral prostaglandin (PGE2) therapy for chronic viral hepatitis B and C. J. Viral Hepatitis 6:329-336. [DOI] [PubMed] [Google Scholar]
- 25.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 26.Katori, M., and M. Majima. 2000. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm. Res. 49:367-392. [DOI] [PubMed] [Google Scholar]
- 27.Kawai, S. 1998. Cyclooxygenase selectivity and the risk of gastro-intestinal complications of various non-steroidal anti-inflammatory drugs: a clinical consideration. Inflamm. Res. 47(Suppl. 2):S102-S106. [DOI] [PubMed] [Google Scholar]
- 28.Khyatti, M., and J. Menezes. 1990. The effect of indomethacin, prostaglandin E2 and interferon on the multiplication of herpes simplex virus type 1 in human lymphoid cells. Antivir. Res. 14:161-172. [DOI] [PubMed] [Google Scholar]
- 29.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 30.Kline, J. N., G. M. Hunninghake, B. He, M. M. Monick, and G. W. Hunninghake. 1998. Synergistic activation of the human cytomegalovirus major immediate early promoter by prostaglandin E2 and cytokines. Exp. Lung Res. 24:3-14. [DOI] [PubMed] [Google Scholar]
- 31.Kujubu, D. A., B. S. Fletcher, B. C. Varnum, R. W. Lim, and H. R. Herschman. 1991. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 266:12866-12872. [PubMed] [Google Scholar]
- 32.Kundu, N., Q. Yang, R. Dorsey, and A. M. Fulton. 2001. Increased cyclooxygenase-2 (cox-2) expression and activity in a murine model of metastatic breast cancer. Int. J. Cancer 93:681-686. [DOI] [PubMed] [Google Scholar]
- 33.Kurland, J. I., and R. Bockman. 1978. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J. Exp. Med. 147:952-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lara-Pezzi, E., M. V. Gomez-Gaviro, B. G. Galvez, E. Mira, M. A. Iniguez, M. Fresno, A. C. Martinez, A. G. Arroyo, and M. Lopez-Cabrera. 2002. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J. Clin. Investig. 110:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau, C. K., W. C. Black, M. Belley, C. Chan, S. Charleson, D. Denis, J. Y. Gauthier, R. Gordon, D. Guay, P. Hamel, S. Kargman, Y. Leblanc, J. Mancini, M. Ouellet, D. Percival, P. Prasit, P. Roy, K. Skorey, P. Tagari, P. Vickers, and E. Wong. 1997. From indomethacin to a selective COX-2 inhibitor. Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors. Adv. Exp. Med. Biol. 407:73-78. [PubMed] [Google Scholar]
- 36.Liu, S., I. V. Pavlova, H. W. Virgin, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luczak, M., W. Gumulka, S. Szmigielski, and M. Korbecki. 1975. Inhibition of multiplication of parainfluenza 3 virus in prostaglandin-treated WISH cells. Arch. Virol. 49:377-380. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Guzman, D., T. Rickabaugh, T.-T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto, H., H. Naraba, M. Murakami, I. Kudo, K. Yamaki, A. Ueno, and S. Oh-ishi. 1997. Concordant induction of prostaglandin E2 synthase with cyclooxygenase-2 leads to preferred production of prostaglandin E2 over thromboxane and prostaglandin D2 in lipopolysaccharide-stimulated rat peritoneal macrophages. Biochem. Biophys. Res. Commun. 230:110-114. [DOI] [PubMed] [Google Scholar]
- 40.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mnich, S. J., A. W. Veenhuizen, J. B. Monahan, K. C. Sheehan, K. R. Lynch, P. C. Isakson, and J. P. Portanova. 1995. Characterization of a monoclonal antibody that neutralizes the activity of prostaglandin E2. J. Immunol. 155:4437-4444. [PubMed] [Google Scholar]
- 42.Mocarski, E. S., Jr. 2002. Virus self-improvement through inflammation: no pain, no gain. Proc. Natl. Acad. Sci. USA 99:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2843. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 44.Mori, N., H. Inoue, T. Yoshida, T. Tanabe, and N. Yamamoto. 2001. Constitutive expression of the cyclooxygenase-2 gene in T-cell lines infected with human T cell leukemia virus type I. Int. J. Cancer 94:813-819. [DOI] [PubMed] [Google Scholar]
- 45.Moriuchi, M., H. Inoue, and H. Moriuchi. 2001. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 75:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narumiya, S., Y. Sugimoto, and F. Ushikubi. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193-1226. [DOI] [PubMed] [Google Scholar]
- 48.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gamma-herpesvirus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neyts, J., and E. De Clercq. 1998. In vitro and in vivo inhibition of murine gammaherpesvirus 68 replication by selected antiviral agents. Antimicrob. Agents Chemother. 42:170-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Neill, G. P., B. P. Kennedy, J. A. Mancini, S. Kargman, M. Ouellet, J. Yergey, J. P. Falgueyret, W. A. Cromlish, P. Payette, C. C. Chan, et al. 1995. Selective inhibitors of COX-2. Agents Actions Suppl. 46:159-168. [DOI] [PubMed] [Google Scholar]
- 51.Ongradi, J., and A. Telekes. 1990. Relationship between the prostaglandin cascade and virus infection. Acta Virol. 34:380-400. [PubMed] [Google Scholar]
- 52.Palumbo, G. J., W. C. Glasgow, and R. M. Buller. 1993. Poxvirus-induced alteration of arachidonate metabolism. Proc. Natl. Acad. Sci. USA 90:2020-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portanova, J. P., Y. Zhang, G. D. Anderson, S. D. Hauser, J. L. Masferrer, K. Seibert, S. A. Gregory, and P. C. Isakson. 1996. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 184:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pyeon, D., F. J. Diaz, and G. A. Splitter. 2000. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyo, H., H. Choy, G. P. Amorino, J. S. Kim, Q. Cao, S. K. Hercules, and R. N. DuBois. 2001. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin. Cancer Res. 7:2998-3005. [PubMed] [Google Scholar]
- 56.Reddy, R. C., G. H. Chen, K. Tateda, W. C. Tsai, S. M. Phare, P. Mancuso, M. Peters-Golden, and T. J. Standiford. 2001. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L537-L543. [DOI] [PubMed] [Google Scholar]
- 57.Reddy, S. T., and H. R. Herschman. 1994. Ligand-induced prostaglandin synthesis requires expression of the TIS10/PGS-2 prostaglandin synthase gene in murine fibroblasts and macrophages. J. Biol. Chem. 269:15473-15480. [PubMed] [Google Scholar]
- 58.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 59.Santoro, M. G. 1997. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 5:276-281. [DOI] [PubMed] [Google Scholar]
- 60.Savard, M., C. Belanger, M. J. Tremblay, N. Dumais, L. Flamand, P. Borgeat, and J. Gosselin. 2000. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J. Immunol. 164:6467-6473. [DOI] [PubMed] [Google Scholar]
- 61.Smith, W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 62.Speir, E., Z. X. Yu, V. J. Ferrans, E. S. Huang, and S. E. Epstein. 1998. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 83:210-216. [DOI] [PubMed] [Google Scholar]
- 63.Steer, S. A., J. M. Moran, L. B. Maggi, Jr., R. M. Buller, H. Perlman, and J. A. Corbett. 2003. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J. Immunol. 170:1070-1076. [DOI] [PubMed] [Google Scholar]
- 64.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 65.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 66.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 67.Taketo, M. M. 1998. COX-2 and colon cancer. Inflamm. Res. 47(Suppl. 2):S112-S116. [DOI] [PubMed] [Google Scholar]
- 68.Terry, L. A., J. P. Stewart, A. A. Nash, and J. K. Fazakerley. 2000. Murine gammaherpesvirus-68 infection of and persistence in the central nervous system. J. Gen. Virol. 81:2635-2643. [DOI] [PubMed] [Google Scholar]
- 69.Thivierge, M., C. Le Gouill, M. J. Tremblay, J. Stankova, and M. Rola-Pleszczynski. 1998. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92:40-45. [PubMed] [Google Scholar]
- 70.Ushikubi, F., E. Segi, Y. Sugimoto, T. Murata, T. Matsuoka, T. Kobayashi, H. Hizaki, K. Tuboi, M. Katsuyama, A. Ichikawa, T. Tanaka, N. Yoshida, and S. Narumiya. 1998. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395:281-284. [DOI] [PubMed] [Google Scholar]
- 71.Vane, J. R., and R. M. Botting. 1998. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 47(Suppl. 2):S78-S87. [DOI] [PubMed] [Google Scholar]
- 72.Virgin, H. W., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wachsman, M., L. Aurelian, and J. W. Burnett. 1990. The prophylactic use of cyclooxygenase inhibitors in recurrent herpes simplex infections. Br. J. Dermatol. 123:375-380. [DOI] [PubMed] [Google Scholar]
- 74.Wadleigh, D. J., S. T. Reddy, E. Kopp, S. Ghosh, and H. R. Herschman. 2000. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 275:6259-6266. [DOI] [PubMed] [Google Scholar]
- 75.Wu, T. T., L. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu, T. T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie, W. L., J. G. Chipman, D. L. Robertson, R. L. Erikson, and D. L. Simmons. 1991. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. USA 88:2692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]