Abstract
In Alzheimer's disease (AD) neurons suffer dysfunction and death associated with aberrant tau phosphorylation and subsequent neurofibrillary tangles. A new study reveals a surprising neuroprotective role for a truncated p73 isoform (ΔNp73). Aged mice with reduced ΔNp73 levels exhibit tau pathology and cognitive deficits, and ΔNp73 reduction in mice with amyloid pathology causes extensive tangle formation and neuron death. These findings provide a novel animal model of AD and a potential therapeutic role for ΔNp73 inducers.
ΔNp73 deficiency promotes neuronal degeneration and cognitive impairment
The memory impairment that occurs in Alzheimer's disease (AD) is caused by the dysfunction and death of neurons in the hippocampus and associated brain regions. As the neurons degenerate they develop neurofibrillary tangles which are intracellular accumulations of hyperphosphorylated tau, a microtubule-associated protein (1). Tau tangles also occur in frontotemporal lobe dementias (FTD) and to a much lesser extent in normal aging. The molecular alterations responsible for tangle formation are believed to include aberrant activation of kinases and inhibition of phosphatases, although the details remain unresolved. Now Wetzel et al. (2) provide evidence that ΔNp73 protects neurons against tau hyperphosphorylation and tangle formation, and preserves cognitive ability during aging and in AD. ΔNp73 is a truncated form of p73, a member of the p53 family of transcription factors (see Text Box 1); ΔNp73 is expressed in neurons and has been suggested to participate in cell differentiation and survival (3). Wetzel et al. found that mice with reduced ΔNp73 levels (Tp73+/− mice) show symptoms of cognitive impairment and motor dysfunction as they age, which are associated with reductions in the size of the hippocampus, motor cortex and cerebellum. Antibodies that recognize a phosphorylated form of tau peculiar to neurofibrillary tangles in AD and FTD, stained many neurons in the brains of Tp73+/− mice but not in normal wild-type mice. They next asked whether ΔNp73 might also protect neurons against the tau pathology and degeneration in AD. To do so they crossed Tp73+/− mice with mice that express a mutant form of β-amyloid precursor protein (APP) that causes inherited AD in humans (2). The APP mutant mice develop progressive accumulation of amyloid β-peptide (Aβ) in their brains and cognitive impairment due to synaptic dysfunction, but no tau pathology or neuron death (4). By contrast, the APP mutant mice with reduced ΔNp73 levels exhibited abundant phospho-tau immunoreactive neurons in the lateral and frontal cortices and hippocampus, and neuron loss in the primary motor cortex. The Aβ pathology was unaffected by levels of ΔNp73 in the APP mutant mice, suggesting that ΔΝp73 protects neurons against Aβ-induced neurofibrillary degeneration (Figure 1A).
Text Box 1.
p53 is a tumor suppressor protein that mediates apoptosis, a form of programmed cell death which occurs during development and normal turnover of cells in tissues, but might also play a pivotal role in disease processes including neurodegenerative disorders. Full-length p73 might also function as a pro-apoptotic protein, but ΔNp73 antagonizes p53 and full-length p73 and protects neurons against death (3). In neurons p53 can be induced by DNA damage, overactivation of glutamate receptors and exposure to neurotoxic oligomeric forms of Aβ (20, 21). p53 kills neurons by inducing the expression of pro-apoptotic proteins including Bax and p21, and might also act directly on mitochondria to increase membrane permeability and cytochrome c release (Figure 1B). p53 levels are increased in neuronal cell bodies and neurites with tau pathology as well as in dystrophic neurons associated with Aβ plaques in AD patients (22). Treatment with a synthetic p53 inhibitor protects neurons against Aβ-induced death, suggesting a pivotal role for p53 in neuronal degeneration in AD (23). A cause – effect relationship between p53 and tau tangle formation was suggested from the observation that p53 overexpression results in tau hyperphosphorylation in cultured cells (24). In the latter study p53 was located in the nucleus, whereas tau was in the cytosol, indicating that the effect of p53 on tau phosphorylation was indirect. The new findings from Wetzel et al. suggest that ΔNp73 might normally antagonize p53-dependent tau tangle-promoting effects (2).
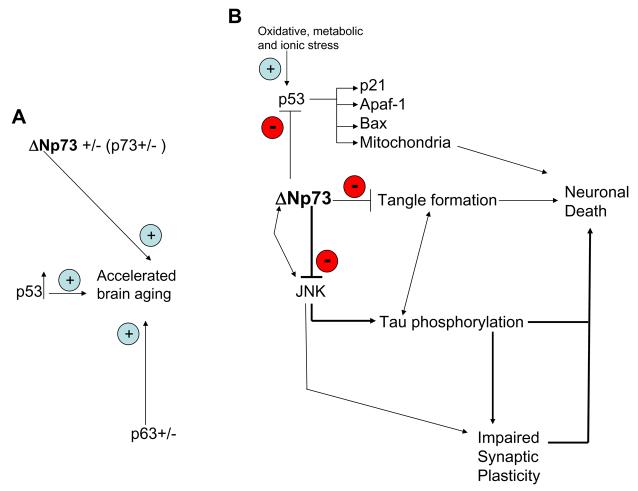
Figure 1.
Possible pathways by which ΔNp73 modifies brain aging and the pathogenesis of Alzheimer's disease. By acting as a negative regulator of p53 and by directly inhibiting JNK ΔNp73 protects neurons against degeneration. A. Overexpression of p53 as well as deletion of one allele of Tp63 or one allele of ΔNp73 causes accelerated aging and neurodegeneration. B. Downstream targets of ΔNp73. ΔNp73 inhibits p53 activity and this reduces the levels of Bax, p21 and Apaf-1. ΔNp73 interacts with JNK and inhibits its activity resulting in reduced tau phosphorylation, decreased neuronal vulnerability and preserved synaptic plasticity. As p53 levels are not changed in Tp73+/− mice, the major pathway is probably via JNK. Reduced levels of ΔNp73 in the Tp73+/− mice or in AD removes the inhibition of ΔNp73 on JNK and p53 activities thereby triggering neuronal death. These pathways can partially explain how ΔNp73 might preserve cognitive ability during aging and in AD.
ΔNp73 prevents tangle formation by inhibiting JNK
The mechanism by which p73 prevents tangle formation has not been established, but clues are provided by the present and previous findings from the Kaplan and Miller laboratories. They showed that ΔNp73 can protect neurons against death by preventing the upregulation of the p53 gene targets p21 and Apaf-1, and by inhibiting c-Jun N-terminal kinase (JNK) activation (5). By studying dorsal root ganglion neurons cultured from neonatal and adult mice, they showed that ΔNp73 plays a pivotal role in protecting adult neurons against death induced by neurotrophin deprivation and DNA damage (6). Wetzel et al. (2) found that levels of p53 and its downstream target p21 did not differ in brain tissue samples from wild-type and Tp73+/− mice. Instead, they found that levels of activated JNK were increased in Tp73+/− brain cells. As ΔNp73 can directly interact with JNK and inhibit its activity (5), the authors tested whether JNK was responsible for tau hyperphosphorylation in the brains of aged Tp73+/− mice and APP mutant mice with reduced p73 levels. They found that treatment of cortical neurons from Tp73−/− and wild-type mice with a JNK inhibitor decreased phosphorylated tau levels (2). A working model can therefore be proposed in which ΔNp73 protects the brain against aging- and AD-related tau pathology and consequent neuronal dysfunction and cognitive impairment by suppressing JNK activity and antagonizing the pro-apoptotic actions of p53 (Figure 1). However, because other kinases including glycogen synthase kinase-3β and cyclin-dependent protein kinase-5 are implicated in pathological tau phosphorylation (1), it will important to establish the relative contributions of each of the different kinases to the AD process. Moreover, it will be interesting to determine if JNK inhibition in vivo also reduces cognitive and behavioral symptoms and neuronal death.
p53 family members, synaptic plasticity and Alzheimer's disease
Although Wetzel et al. (2) reveal a previously unknown role for ΔNp73 in protecting neurons against tau pathology, their findings must be reconciled with other data concerning the normal functions of p73 and its involvement in AD. Wilson et al. (7) found that p73 accumulates in the nucleus, and is also present in neurofibrillary tangles and dystrophic neurites in the brains of AD patients. However, they did not establish whether the p73 immunoreactivity present in the tangle-bearing neurons was full-length p73 or ΔNp73. Indeed, another study provided evidence that ΔNp73 expression is decreased in AD (8). A concomitant increase in full-length p73 levels and decreased ΔNp73 levels would have devastating consequences if full-length p73 induces the expression of pro-apoptotic genes and ΔNp73 normally blocks JNK-mediated tau hyperphosphorylation.
Is tau tangle formation a critical alteration caused by a decreased ΔNp73 : p73 ratio that results in cognitive deficits in aging and AD? The fact that most lines of APP mutant mice exhibit age-related accumulation of Aβ and memory deficits in the absence of any detectable tau pathology argues against a critical role for tau (4). Even in 3xTgAD mice that develop both Aβ and tau pathologies, memory can be preserved by environmental manipulation (food deprivation on alternating days) that does not retard the progression of the tau pathology (9). Perhaps a tau-independent effect of ΔNp73 deficiency accounts for impaired synaptic plasticity and memory deficits during aging and in AD (Figure 1B). Wetzel et al. (2) did not evaluate cognitive function in the APP x p73+/− mice; if cognitive function was worsened in these mice, this would suggest that individuals that are haploinsufficient for p73 may be at higher risk for early onset AD or a more severe form of AD
Data from studies of cultured neural cells suggest that full-length p73 promotes, and ΔNp73 antagonizes, neuron differentiation (10). The prominent hippocampal neuronal apoptosis and atrophy present in mice lacking both p73 and ΔNp73 (11) is apparently due to ΔNp73 deficiency, whereas hippocampal dysgenesis in the full p73 knockout is due to lack of TAp73 (12). The cognitive impairment in p73+/− mice, suggest an important role for ΔNp73 in neuronal plasticity (2). It will therefore be important to determine if and how p53 family members influence synaptic plasticity in the adult during aging and in AD. The most obvious way in which p53, p73 and p63 could affect plasticity is by regulating the expression of genes encoding proteins that regulate synaptic transmission or structural remodeling. For example, some evidence suggests that several p53 targets (13) regulate plasticity including Wnt7b which regulates dendritic growth (14) and Bax which might enhance neurotransmitter release (15). By contrast, p53 is present in synaptic terminals where it can mediate mitochondrial alterations caused by oxidative and excitotoxic stress (16). It will therefore be of interest to determine if p73 and/or ΔNp73 are also present in synapses and modulate synaptic plasticity and memory by transcription-independent mechanisms. The evidence that ΔNp73 directly interacts with JNK to reduce its kinase activity (2, 5) opens the possibility that the activities of other enzymes involved in neuronal plasticity and survival are regulated by ΔNp73 or other p53 family members.
Concluding remarks: where do we go from here?
The intriguing findings of Wetzel et al.(2) suggest many questions. What factors influence the expression and biological activities of ΔNp73 in the contexts of neuronal plasticity and degeneration? Does ΔNp73 participate in neurological disorders other than AD? Three alterations that are believed to participate in the early synaptic dysfunction and subsequent neuronal degeneration in aging and AD are increased oxidative stress, impaired energy metabolism and perturbed cellular Ca2+ homeostasis (17). These adverse conditions can induce p53 expression and activate JNK, but their effects on ΔNp73 levels and activities are unknown. Perhaps ΔNp73 function(s) is inhibited by oxidative/metabolic stress or excessive Ca2+ accumulation in neurons, caused by the aging process and Aβ, thereby promoting synaptic dysfunction and neuronal degeneration. JNK and p53 are implicated in neuronal death in Parkinson's and Huntington's diseases (18, 19), and it will therefore be of considerable interest to determine the effects of ΔNp73 overexpression in animal models of these disorders. The potential for manipulations that increase ΔNp73 levels or drugs that enhance its neuroprotective actions merit preclinical investigation, and it will be of interest to determine whether elevated ΔNp73 levels preserve cognitive ability during aging and in AD. In this regard, the APP; Tp73+/− double mutant mice provide a novel preclinical model that recapitulates all of the major abnormalities of AD patients including Aβ and tau pathologies, neuronal death and memory impairment.
References
- 1.Iqbal K, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Wetzel MK, et al. p73 regulates neurodegeneration and phospho-tau accumulation during aging and Alzheimer's disease. Neuron. 2008;59:708–721. doi: 10.1016/j.neuron.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs WB, Walsh GS, Miller FD. Neuronal survival and p73/p63/p53: a family affair. Neuroscientist. 2004;10:443–455. doi: 10.1177/1073858404263456. [DOI] [PubMed] [Google Scholar]
- 4.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Lee AF, Ho DK, Zanassi P, Walsh GS, Kaplan DR, Miller FD. Evidence that DeltaNp73 promotes neuronal survival by p53-dependent and p53-independent mechanisms. J. Neurosci. 2004;24:9174–9184. doi: 10.1523/JNEUROSCI.1588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh GS, Orike N, Kaplan DR, Miller FD. The invulnerability of adult neurons: a critical role for p73. J. Neurosci. 2004;24:9638–9647. doi: 10.1523/JNEUROSCI.1299-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson C, Henry S, Smith MA, Bowser R. The p53 homologue p73 accumulates in the nucleus and localizes to neurites and neurofibrillary tangles in Alzheimer disease brain. Neuropathol. Appl. Neurobiol. 2004;30:19–29. doi: 10.1046/j.0305-1846.2003.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, et al. TP73 allelic expression in human brain and allele frequencies in Alzheimer's disease. BMC Med. Genet. 2004 Jun 2;5:14. doi: 10.1186/1471-2350-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halagappa VK, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 10.De Laurenzi V, et al. Induction of neuronal differentiation by p73 in a neuroblastoma cell line. J. Biol. Chem. 2000;275:15226–15231. doi: 10.1074/jbc.275.20.15226. [DOI] [PubMed] [Google Scholar]
- 11.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 12.Tomasini R, et al. Tap73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brynczka C, Labhart P, Merrick BA. NGF-mediated transcriptional targets of p53 in PC12 neuronal differentiation. BMC Genomics. 2007;8:139. doi: 10.1186/1471-2164-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ros SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 15.Jonas EA, Hardwick JM, Kaczmarek LK. Actions of BAX on mitochondrial channel activity and on synaptic transmission. Antioxid. Redox Signal. 2005;7:1092–1100. doi: 10.1089/ars.2005.7.1092. [DOI] [PubMed] [Google Scholar]
- 16.Gilman CP, Chan SL, Guo Z, Zhu X, Greig N, Mattson MP. p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults. Neuromolecular Med. 2003;3:159–172. doi: 10.1385/NMM:3:3:159. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr. Pharm. Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 19.Scappini E, Koh TW, Martin NP, O'Bryan JP. Intersectin enhances huntingtin aggregation and neurodegeneration through activation of c-Jun-NH2-terminal kinase. Hum. Mol. Genet. 2007;16:1862–1871. doi: 10.1093/hmg/ddm134. [DOI] [PubMed] [Google Scholar]
- 20.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J. Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Monte SM, Sohn YK, Wands JR. Correlates of p53- and Fas (CD95)-mediated apoptosis in Alzheimer's disease. J. Neurol. Sci. 1997;152:73–83. doi: 10.1016/s0022-510x(97)00131-7. [DOI] [PubMed] [Google Scholar]
- 23.Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J. Neurochem. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- 24.Hooper C, Meimaridou E, Tavassoli M, Melino G, Lovestone S, Killick R. p53 is upregulated in Alzheimer's disease and induces tau phosphorylation in HEK293a cells. Neurosci. Lett. 2007;418:34–37. doi: 10.1016/j.neulet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]