Abstract
Background
Malt liquor (ML) beverages have become increasingly popular among urban minority groups, due partly to their inexpensive price and targeted advertising. We hypothesized that non-fermented by-products contained in ML beverages will alter the pharmacokinetics (PK) and pharmacodynamic (PD) effects of its ethanol content. In addition, we determined the effect of alcohol dehydrogenase (ADH) genotypes on the PK following consumption of malt liquor beverages.
Methods
The study was conducted in 31 healthy adult African-American social drinkers, mean ±SD age of 22.3±1.3 years, and weight of 70.7±10.9 kg. Participants were administered ethanol, in randomized order, two-weeks apart, in the form of oral ML beverage (6%v/v), or isocaloric solution of diet soda–ethanol (DS-Etoh) beverage (6%v/v). During each session the beverage was consumed over 4 minutes and breath alcohol concentrations (BrAC) as well as subjective and behavioral effects of ethanol were evaluated over 180 minutes. Pharmacokinetic parameters of ethanol were calculated using Michaelis-Menten elimination kinetics. The effect of ADH genotype on PK was evaluated using the Wilcoxon Signed rank test.
Results
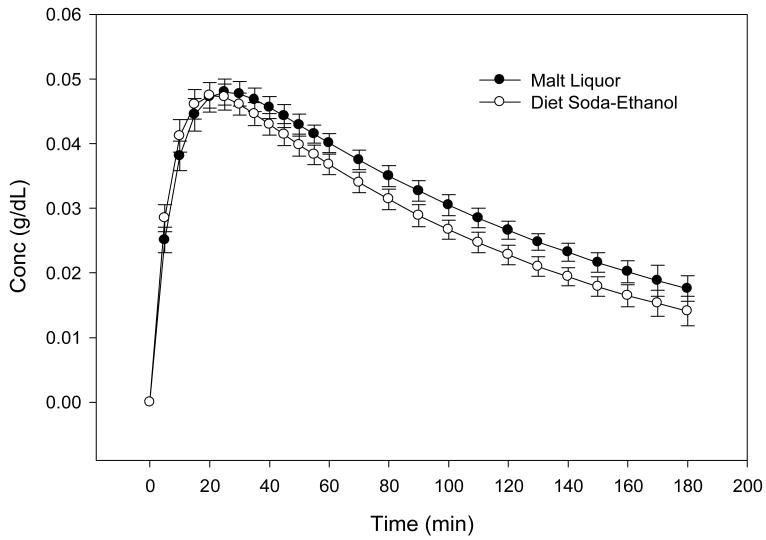
Results show a slower mean rate of absorption, Ka, (0.12 vs. 0.15 min−1, p=0.03) and a longer time to reach maximum concentration, Tmax, (28 vs. 23 min. p<0.01) for the ML compared to DS-Etoh beverage. The ML beverage resulted in a larger area under the BrAC-time curve compared to DS-Etoh beverage (8.4 vs. 6.8 min*g/dL, p=0.02). There was no difference in the subjective PD effects between the 2 beverages.
Conclusion
Results show that exposure to ethanol following consumption of ML beverages is different compared to that following non-malt beverages in African Americans. These differences may be related to non-fermented by-products present in commercially available ML products. These PK differences do not appear to result in significant perceived alcohol PD changes, nor are they related to ADH genotype.
Keywords: malt liquor, pharmacokinetics, alcohol dehydrogenase, aldehyde dehydrogenase, African-Americans
INTRODUCTION
Malt liquor is becoming increasingly popular among college students, although it is primarily sold in low-income areas. Insufficient information is known regarding beverage preference, specifically among understudied inner-city minority populations (Graves & Kashkutas, 2002). Consequently, most researchers do not define clear differences between malt liquor and beer or among wine, fortified wine, and wine coolers. Research on beverage-specific prototypes is important because it informs the epidemiology of consumption, assists in identifying population groups at risk for specific alcohol-related problems, and highlights targeted communities subject to disproportionate advertising and distribution (Bluthenthal, et al, 2005; Graves & Kashkutas, 2002). In the United States, there is increasing public interest in malt liquor beverage consumption among young people and ethnic minority populations (Alaniz and Wilkes, 1998).
Malt liquor refers to a beverage fermented from yeast that has an alcohol content between 5.6–8% w/v (Kerr & Greenfield, 2003). It contains non-fermented by-products ("real extract") that produce much of the taste of these beverages. Ingredients such as antioxidants and tannins are added to produce a harsh taste. Malt liquor has a relatively low cost per ounce of absolute alcohol and is often packaged in large-volume (40 ounce and larger), sometimes non-recloseable containers that encourage more immediate and continuous consumption (Kerr & Greenfield, 2003). These products may have a higher alcohol content than regular beer or wine coolers which typically contain no more than 5% alcohol and are usually sold in 12 ounce and smaller containers. In addition to their inexpensive cost, the popularity of these high alcohol content malt liquor beverages may be attributed to aggressive and targeted marketing (Moore, DJ et al, 1996; LaVeist & Wallace, 2000). Consumption of these low cost, higher alcoholic content malt beverages and other sweetened alcoholic beverages, especially among adolescents and young adults, is of concern because they may contribute to increased alcohol use and abuse and increased risk to develop dependency.
The biological basis for the popularity of these malt beverages has not been systematically studied. One explanation may be related to pharmacokinetic (PK) "enhancement" of ethanol absorbed from these beverages by the presence of by-products found in these malt beverages, including oligosaccharides derived from the amylolytic breakdown of amylase and amylopectin, partially degraded proteins, various minerals and minor organics of plant origin.
There have been a limited number of studies that describe alcohol PK after the consumption of beer or other malt derived beverages (Greenfield et al 2004; Bluthenthal et al 2005). Most utilize the “oral alcohol challenge” method which has been widely used to study the PK of ethanol under a variety of experimental conditions (Thomasson et al 1995, Jones and Jonsson 1994, Wang et al 1992, Mumenthaler et al 2000, Jones et al 1997, Whitfield and Martin 1994, Jones et al 1992). Variability in response using the oral challenge methods result from such critical issues as sample size, genetic polymorphism in alcohol metabolic rates, food intake before ethanol ingestion, and use of appropriate controls (Holford, 1997). These issues may explain inter- and intra-individual differences in the relative bioavailability and complex PK after the consumption of beverages with differing ethanol concentrations.
The focus of the current study was to evaluate differences in alcohol PK following administration of a dose of ethanol in a fixed concentration in a commercially available malt liquor beverage compared to the same dose of ethanol in the same fixed concentration administered in a non-malt vehicle where the "real extract" was absent. In particular, this study examined in a single-blind, crossover design, whether malt liquor formulations result in higher peak alcohol levels, a greater rate of rise in the ascending limb of the alcohol concentration-time curve, or a greater area under the BrAC-time curve (AUC) in a cohort of young adult African American social drinkers without a personal or family history of alcohol dependence. A single-blind crossover design was used to allow within-participant comparison, since each participant served as their own and provide an unbiased difference in alcohol effect, if any. In addition, differences in the subjective effects of ethanol were examined. A secondary focus was to examine the effect of the alcohol dehydrogenase (ADH) genotype on the PK of the ethanol following consumption of malt liquor. Examining the effect of ADH genotype on the PK of alcohol in a segment of the African American population with a preference for alcohol beverages like malt liquor may provide insight as to why this preference exists.
METHODS
Participants
Data were obtained from young adult African-American paid volunteers who read and signed an informed consent statement, approved by the Howard University Institutional Review Board (IRB) and who met inclusion /exclusion criteria for enrollment in the malt liquor study. Subjects were social drinkers without self-reported alcohol problems. Inclusion criteria included: (1) age between 21–25 years, (2) height of 152–188 cm and weight of 68–80 kg for males and 52–64 kg for females, (3) absence of major medical problems based on a medical history and physical examination, (4) results within normal limits for the following serum lab tests: complete blood count (CBC), alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase, creatinine, glucose and Hepatitis C, and a negative urine pregnancy test for females. Exclusion criteria included a history of renal, pulmonary, gastrointestinal, liver, heart disease; or a psychiatric diagnosis or prescribed psychotropic medications. Participants completed the Timeline Followback questionnaire to obtain drinking history over the past thirty days (Sobell and Sobell, 1995). Thirty one subjects started and completed the trial.
Experimental Procedure
Subjects participated in two experimental sessions in a single blind, within-subject crossover design. During each session, eligible participants were admitted to the Howard University General Clinical Research Center at 8:00AM, following an overnight fast. Baseline data was obtained on all participants, including height, weight, a brief medical history and a urine pregnancy test for females.
Preparation and Administration of Alcoholic Beverages
At approximately 9:00 AM, subjects received in a randomized order the following alcoholic beverages during each session:
Malt Liquor (ML)
the same commercially available Olde English brand malt liquor beverage containing 6% v/v ethyl alcohol was used in all studies. Olde English brand was chosen because of its easy availability.
Diet soda-Ethanol (DS-Etoh)
Diet soda with 95% alcohol diluted to 6% v/v and made isocaloric with the addition of sucrose. Diet Sprite was used because of its low caloric content and absence of food coloring agents.
For each subject, the beverages were prepared based on their total body water volume, estimated from their height and weight measurements in addition to gender and age. A calculated test volume of the ML or DS-Etoh required to reach a peak concentration of 50mg%, was prepared utilizing a normogram based on the estimated total body volume (Watson et al, 1980).
A ten (10) ml sample of the alcohol beverage to be administered was taken for analysis of the alcohol content. The temperature of the alcohol beverage (ranging from 44–46 °F) was also recorded. The total volume of the beverage was divided into three equal aliquots and the subjects were instructed to consume each aliquot over one minute time period with a thirty-second interval between consuming each aliquot. Each subject was required to complete consumption over a total of 4 minutes. One participant did not consume the beverage during the allotted time interval and data from that subject was excluded from analysis. Immediately thereafter, the subjects underwent six, vigorous mouth rinses of 20 seconds duration, each utilizing 30 ml of tepid tap water. This was done in order to decrease artificially high breath alcohol concentration (BrAC), from residual ethanol in the buccal cavity. The final mouth rinse was followed by a waiting interval of three minutes before the first procedural BrAC was done (10 min after the start of dosing). Serial BrAC were evaluated over 180 minutes, utilizing a calibrated Alcotest Breathalyzer 7410, a hand held meter device (Drager Safety, Durango, CO). The BrAC was recorded every 5 minutes for the first hour and then every 10 minutes until the end of the session. Subjects were continually monitored for adverse reactions including but not limited to nausea, vomiting, headache, flushing and rashes during the study session. All subjects were offered apple sauce, saltine crackers and apple juice at the termination of the session.
Additionally, serial measurements of subjective perceptions were collected using self-report questionnaires, with the first taken 30 minutes prior to administration of the beverage, then starting 15 minutes after time of consumption and every 15 minutes for the first two hours and then every 30 minutes until the end of the session. The questionnaires included 2 items derived from the Subjective High Assessment Scale (SHAS) [Schuckit, 1980], and the Biphasic Alcohol Effects Scale (BAES) [Earlywine and Erbich, 1996]. SHAS is a paper and pencil test in which participants use markings and scales to measure the subjective “high” and “intoxication” effects of alcohol. SHAS is measured as 100 + distance between left edge of the scale to the mark made by the participant on the scale (in mm). BAES is a paper and pencil scale that measures feelings of “sedation” and “stimulation”. The stimulation subscale has 7 items including elated, energized, excited, stimulated, talkative, up, and vigorous, each measured on an 11-point scale (from 0 to 10). Similarly, the sedation subscale has 7 items including difficulty concentrating, down, heavy, inactive, sedated, slow and sluggish, each measured on an 11-point scale (from 0 to 10). If the meaning of a word is unclear to the participant, they are provided with a list of synonyms which can be used to describe the unclear word. The BAES has been shown to be differentially sensitive to the stimulating and sedating effects of alcohol (Martin et al., 1993).
Pharmacokinetic Analysis
The BrAC vs. time data for each session was analyzed separately. The peak concentration, Cmax, and time of peak concentration (Tmax) were obtained directly from the observed data. The area under the breath alcohol concentration-time curve (AUC) was estimated by linear interpolation using the trapezoidal rule. A one-compartment open model with first order oral absorption and Michaelis-Menten elimination kinetics was used to estimate the critical pharmacokinetic parameters (absorption rate, Ka, maximum elimination rate, Vm, Michaelis-Menten constant, Km, volume of distribution, Vd). The appropriateness of the Michaelis-Menten model for alcohol pharmacokinetics has been reviewed in the literature [Dubowski, 1985, Lunquist and Wolthers, 1958]. Nevertheless, the adequacy of the model to the present data was assessed by least squares goodness-of-fit criteria. Analysis was done using the WINNONLIN (Professional Version 3.3) program.
Pharmacodynamic Analysis
The pharmacodynamic effect of ML was assessed primarily by self-reported subjective and behavioral effects of alcohol over 3 hours using the SHAS questionnaires for perceived “high” and “intoxication” effects and BAES for “stimulating” and “sedating” effects. Individual items on the “stimulating” and “sedating” subscales were separately and equally weighted and the results expressed as the mean score per item for each of the subscales. A composite score was calculated as the average of the scores in these items to represent “stimulation” and “sedation” effect.
ADH Genotyping
A venous blood sample was obtained from each subject and DNA was isolated for genotyping. Polymerase chain reaction was used to amplify the significant portions of the ADH locus using amplification primers designed based on the sequence of each gene. This was followed by hybridization with allele-specific radiolabeled oligonucleotide probes. [Yu et al, 1988]
Statistical Analysis
For each subject, the PK parameters were estimated for each session. Standard summary statistics were reported for all PK parameters. The adequacy of the PK model to the data was assessed by residual analysis via least squares goodness-of-fit criteria. The pharmacokinetic parameter estimates were examined univariately and graphically to determine their distributional characteristics. Differences between ML and DS-Etoh PK parameters were tested using a Wilcoxon rank-sum test. Repeated measures analysis of variance (ANOVA) was used to examine the difference in response of subjective measures of “high”, “intoxication”, “sedation” and “stimulation” between ML and DS-Etoh. The effect of the ADH genotype on PK parameters was evaluated using the Wilcoxon signed rank test. The level of statistical significance was set at 0.05. Statistical analysis was conducted using SAS Version 9.1 (SAS Institute, Cary, NC).
Results
Pharmacokinetics
Thirty-one African-American individuals, 55% males with mean age of 22.3 ± 1.3 years completed the two-session crossover study. Baseline characteristics of the study participants are shown in Table 1. Participants were social drinkers, who consumed an average of 12 ±7 drinks per month. Figure 1 depicts the mean BrAC-time profile measured after the oral administration of 6% ML and DS-Etoh. Result of residual analyses, to assess goodness-of-fit of the Michaelis-Menten model to the data is presented in Table 2. The correlation between observed and predicted data ranged from 0.84 to 0.99. Measures of residuals are all less than 10−2, suggesting a convincingly good fit of the model to the data. The estimated mean PK parameters are summarized and compared in Table 3. Comparison of PK parameters showed a slower Ka (0.12 vs. 0.15 min−1, p=0.03), a slower Tmax (28 vs. 23 min., p< 0.01), but a larger area under the BrAC-Time curve (8.4 vs. 6.8 min*g/dL, p=0.02) for the ML compared to the DS-Etoh treatment. The other PK parameters including Vm, Vd and Cmax were not significantly different between treatments (see Table 3). Moreover, as expected, there were no statistical significant difference in PK parameters between males and females.
Table 1.
Baseline Characteristics of the study participants (N=31). Data are mean±SD.
| Variable | Males (n=17) | Females (n= 14) | All (n=31) |
|---|---|---|---|
| Age (yrs) | 22.2 ± 1.2 | 22.6 ± 1.5 | 22.3 ± 1.3 |
| Weight (kg) | 76.0 ± 9.9 | 63.2 ± 7.2 | 70.7 ± 10.9 |
| Height (cm) | 178.3 ± 6.2 | 164.4 ± 5.1 | 172.4 ± 9.0 |
| * Total Drinks/ 30 days | 14.9±7.6 | 9.4±6.0 | 12.2±7.4 |
Total drinks were obtained from the Time Line Follow Back 30 days prior to screening. A standard drink contains 14g of pure alcohol (approximately 0.6 fl oz. or 1.2 tablespoons)
Fig 1. Mean BrAC vs. Time Profiles for Alcohol as Malt Liquor and Diet Soda Ethanol (n=31) Error bars are standard error of the mean.
Total volume of beverage was divided into 3 equal aliquots, with subject consuming each aliquot in 1 minute with a 30 second interval between drinks, followed by 6 vigorous mouth rinses, 20 seconds duration before the first BrAC was taken.
Table 2.
Residual Analysis Assessing Goodness-of-fit of Michaelis-Menten Model. Data are mean [range]
| Malt Liquor | Diet-Soda Ethanol | |
|---|---|---|
| R (obs, pred) | 0.932 [0.841–0.991] | 0.954[0.877–0.991] |
| CSS | 0.0027[0.0006–0.0057] | 0.0032[0.0007–0.0067] |
| SSR | 0.0003[0.0001–0.0013] | 0.0002[0.0001–0.0010] |
| WCSS | 0.0027[0.0006–0.0058] | 0.0032[0.0007–0.0067] |
| WSSR | 0.0003[0.0001–0.0013] | 0.0002[0.0001–0.0010] |
| S | 0.0037[0.0019–0.0084] | 0.0034[0.0017–0.0071] |
R: correlation between observed and predicted data
CSS: corrected sum of squares
WCSS: weighted corrected sum of squares
SSR: sum of square residuals
WSSR: weighted sum of square residuals
S: mean standard deviation
Table 3.
Mean ± S.D. pharmacokinetic parameter estimates for 6% v/v Malt Liquor and 6%v/v Diet-Soda Ethanol (N=31) sessions
| Parameter | Malt Liquor | Diet Soda/Ethanol |
|---|---|---|
| Ka (min−1) | 0.15±0.08 | 0.12±0.04* |
| Vm (min−1) | 0.25±0.03 | 0.26±0.07 |
| Vd (dL) | 387.0±117.5 | 381.0±104.4 |
| Cmax (g* dL−1) | 0.05±0.01 | 0.05±0.01 |
| Tmax (min) | 27.6±8.6 | 22.5± 7.1* |
| AUC (min* gdL−1) | 8.4±3.0 | 6.8±3.2* |
Indicates p < 0.05; Ka indicates absorption rate, Vm maximum elimination rate, Vd volume of distribution, Cmax, peak concentration; Tmax, time to reach peak concentration; AUC, area under the concentration-time curve
Pharmacodynamics
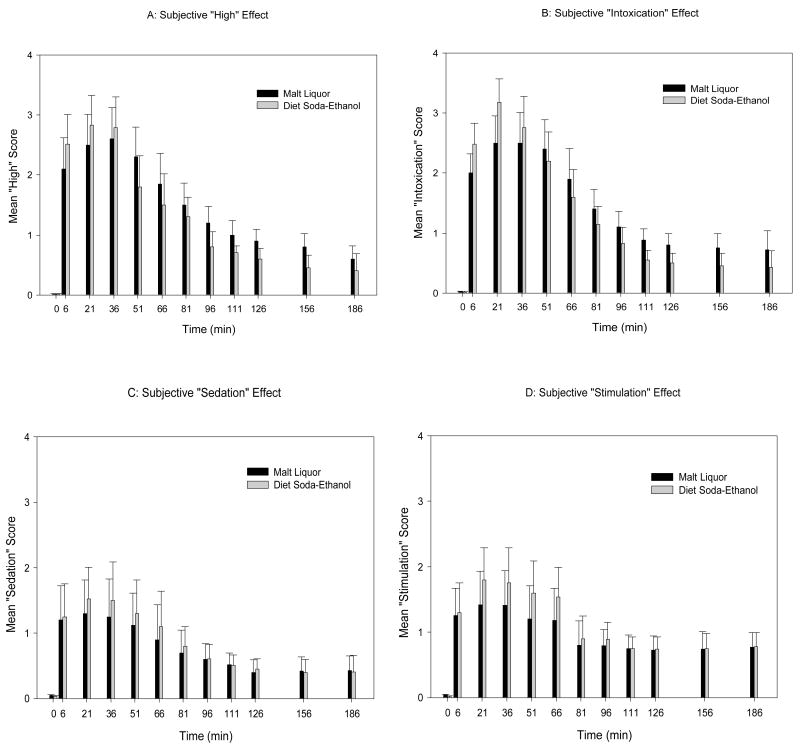
Figures 2A and B, respectively, depict the mean subjective “high” and “intoxication” alcohol effect of ML and DS-Etoh. Although not statistically significant, the DS-Etoh appeared to have higher "intoxication" and "high" effect scores during the ascending limb of the BrAC. The subjective alcohol effects were, however, observed to be pronounced and persistent for the ML group on the descending limb of the BrAC. Figures 2C and D, respectively, depict the mean “sedation” and “stimulation” scores of ML and DS-Etoh. Similarly, there were no statistical difference in “sedation” and “stimulation” effects between ML and DS-Etoh. As with the PK data, there were no difference in subjective measures between males and females.
Fig 2. Subjective response of 6% v/v Malt Liquor and Diet Soda-Ethanol.
(A) Represent subject high response, (B) intoxication effect, (C) sedation effect and (D) stimulation effect. Subjective perceptions were collected using self-reported questionnaires, 30 minutes prior to beverage administration [time 0], then soon after
Genotype
Thirty-one subjects were genotyped for alcohol-metabolism genes at the ADH1B, ADH1C and ALDH2 loci. All participants were homozygous for the ALDH1B*1 allele. Allele and genotype frequencies of the ADH1B and ADH1C loci are presented in Table 3. Sixty-five percent were homozygous for the ADH1C*1 allele and 76% were homozygous for the ADH1B*1 allele. None were homozygous for the ADH1C*2 nor the ADH1B*3 alleles. Table 4 shows the PK of ethanol following consumption of 6% v/v ML for the various ADH genotypes. The results show no significant effect of ADH genetic polymorphisms on the PK parameters.
Table 4.
Allele and Genotype Frequencies of the ADH Polymorphism (N=31)
| ADH1B Polymorphism | ADH1C Polymorphism | ||
|---|---|---|---|
| Alleles (%) | Alleles (%) | ||
| ADH1B*1 | 88.7 | ADH1C*1 | 85.5 |
| ADH1B*3 | 12.3 | ADH1C*2 | 14.5 |
| Genotypes (%) | Genotypes (%) | ||
| ADH1B*1 homozygous | 74.4 | ADH1C*1 homozygous | 71.0 |
| ADH1B*3 homozygous | - | ADH1C*2 homozygous | - |
| ADH1B*1/ADH1B*3 heterozygous | 25.6 | ADH1C*1/ADH1C*2 heterozygous | 29.0 |
Discussion
In the US, approximately 75% of the adult population drinks alcoholic beverages regularly and about 10% are unable to limit their alcohol consumption. Research shows that genetic and biological as well as environmental factors contribute to the overall risk for some people to become alcoholics once they start drinking (Christian et al, 1996; Neale and Martin, 1989; O'Connor et al, 1999; Sorbel et al; 1996; Viken et al 1995). It has been shown that children of alcoholic parents are more likely to develop the disease than children of non-alcoholic parents (Malone et al, 2002). However, anyone who drinks can develop the disease of alcoholism and two of the most significant predictors of an alcohol problem are the quantity and frequency the individual drinks. The significance of beverage type, including malt liquor, as a contributing factor in drinking behavior or alcohol-related problems has not been studied to any significant degree in any population.
This study may be the first to examine the pharmacokinetics of ethanol in malt liquor in a specific population. It was initiated to investigate the PK differences between commercially available malt liquor and other non-malt liquor beverages as well as the effect of the genetic polymorphism of ADH and ALDH2 on pharmacokinetic parameters. Knowing that ethanol pharmacokinetics can be influenced by both genetic and environmental factors, it was felt that the examination of the influence of alcohol metabolism-related genotypes might enhance our understanding of the role of genetic determinants in a segment of the African American population with a preference for alcohol beverages like malt liquor.
In our study, we attempted to carefully utilize the appropriate crossover experimental design and control many of the experimental sources of variability, such as time and duration of administration, time of day, age, ethanol consumption prior to the beginning of the experimental procedures and the fed or fasted state, in a specific population group. The PK literature abounds in emphasis on the large “experimental variance” in the time course of breath and blood alcohol concentrations after oral alcohol administration (Holford, 1987; Pikaar et al, 1988; Freil et al, 1995; Ramchandani et al, 1999). However, the problems as they relate to between subject variations have been studied by Jones and Jonsson, 1994 and Li et al, 2000 and their findings indicate a relatively high degree of within-subject reproducibility in ethanol kinetics when utilizing a crossover design.
In the pharmacokinetic analysis, the ascending limb of the BrAC-Time curve revealed a slower rate of absorption, Ka, and a longer time to reach maximum concentration, Tmax, for the ethanol contained in the ML product when compared to the DS-Etoh (Figure 1). These delays may be due to the substances contained in the ML. The results also revealed a larger area under the curve for the ML compared to the DS-Etoh arm, indicating a greater systemic ethanol exposure following ML compared to DS-Etoh. These results suggest that although the amount of ethanol is the same in both the ML and DS-Etoh beverages, the bioavailability is greater for the ML product when compared to the DS-Etoh.
Although not statistically significant, analysis of the self-reported subjective readings, utilizing the SHAS, showed that the DS-Etoh appeared to have a higher “intoxication” and “high” effect from the baseline to Cmax when compared to the ML (Figures 2a and 2b). This may be secondary to the observed faster absorption of the DS-Etoh as opposed to the ML. The subjective effects on the descending limb of the BrAC-Time curve were observed to be prolonged and persistent for the ML consistent with the greater exposure to the ethanol in the ML product over time.
The analysis of the ADH genotypes revealed a frequency of 0.12 for the ADH1B*3 allele and 0.15 for the ADH1C*3 allele in our study. This is consistent with estimates ranging from 0.15 to 0.25 reported in other studies [Thomasson et al, 1995; Li et al, 2000; Ehrig and Li, 1995]. Although ethanol pharmacokinetics have been shown to be influenced by genetic factors, in our study the presence of the ADH1B*3 allele did not have a significant effect on the PK parameters of the ethanol following the 6%v/v ML preparation. According to other studies, (Li et al, 2001) the allelic variation at the ADH1C locus appears to be in linkage disequilibrium with that at ADH1B, and the polymorphic gene itself does not significantly affect susceptibility to alcohol dependence.
A drawback of the current study is a selection bias, in that participants in the study were limited to African-American young adults. Despite this limitation, the findings of this study demonstrate that there is a difference in the PK of the ethanol contained in the ML versus the DS-Etoh. Although significant, these findings are insufficient to explain the popularity of these commercially available malt liquor beverages. Other factors such as marketing and the targeting of promotional materials to specific segments of the population may also be contributory. The lack of our understanding about the production process and the exact contents of the inert materials inhibit our ability to extend these findings.
Table 5.
Effect of ADH Genotypes on Pharmacokinetic Parameters following ML beverage (N=31)
| ADH1C Polymorphism | ADH1B Polymorphism | |||
|---|---|---|---|---|
| PK Parameter | ADH1C*1 Homozygote (n=22) |
ADH1C*1/ADH1C*2 Heterozygote (n=9) |
ADH1B*3 Homozygote (n=24) |
ADH1B*1/ADH1B*3 Heterozygote (n=7) |
| Cmax (g*dL−1) | 0.05±0.01 | 0.05±0.01 | 0.05±0.01 | 0.06±0.01 |
| Tmax (mins) | 24.0±10.0 | 29.0±8.0 | 25.0±8.0 | 31±16.0 |
| Ka ( min−1) | 0.12±0.06 | 0.12±0.07 | 0.13±0.06 | 0.12±0.05 |
| AUC(min* gdL−1) | 5.2±1.5 | 5.4±1.0 | 5.1±1.2 | 6.2±1.7 |
| Vm (min−1) | 0.09±0.07 | 0.09±0.05 | 0.09±0.07 | 0.11±0.07 |
| Vd (dL) | 384.5±87.4 | 399.6±87.0 | 404.5±81.1 | 333.8±91.6 |
Acknowledgments
We would like to thank Dr. T- K Li for his recommendations on the design of this study.
The study was supported in part by the following grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), R21AA13531, U24AA11898, AA014643, and General Clinical Research Center (GCRC) grant MO1 RR10284.
References
- Alaniz ML, Wilkes C. Pro-drinking messages and message environments for the young adults: the case of alcohol industry advertising in African-American, Latino, and Native American communities. J Public Health Policy. 1998;19:447–72. [PubMed] [Google Scholar]
- Bluthenthal RN, BrownTaylor D, Guzman-Becerra N, Robinson P. Characteristics of Malt Liquor Beer Drinkers in a Low-Income, Racial Minority Community Sample. Alcohol Clin Exp Res. 2005;29:402–409. doi: 10.1097/01.alc.0000156118.74728.34. [DOI] [PubMed] [Google Scholar]
- Christian JC, Corporate S, Norton JA, Williams CJ, O'Connor S, Li T-K. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33:584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Dubowski KM. Absorption, Distribution and Elimination of Alcohol: Highway Safety Aspects. Journal of Studies on Alcohol. 1983;(Suppl 10):8–18. doi: 10.15288/jsas.1985.s10.98. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Erbich J. A confirmed factor structure for the biphasic alcohol effects scale. Exp Clin Psychopharmacol. 1996;4:107–113. [Google Scholar]
- Earleywine CS, Musty M, Perrine RE, Swift RMMW. Development and validation of the Biphasic Alcohol effects Scale. Alcohol Clin Exp Res. 1993;17:135–139. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Ehrig T, Li T-K. Biological Aspects of Alcoholism: Implications for Prevention, Treatment & Policy: Metabolism of Alcohol and Metabolic Consequences. In: Tabakoff, Hoffman, editors. WHO Expert Series on Biol Psy. Vol. 4. Toronto: Hogrefe & Huber, Publishers; 1995. pp. 23–48. [Google Scholar]
- Friel PN, Baer JS, Logan BK. Variability of Ethanol Absorption and Breath Concentrations During a Large-Scale Alcohol Administration Study. Alcohol Clin Exp Res. 1995;19:1055–1060. doi: 10.1111/j.1530-0277.1995.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Bond J, Kerr WC, Korcha R, Brown-Taylor D. Epidemiology of malt liquor beer consumption based on the year 2000 U.S. National Alcohol Survey. Paper presented at the 132nd American Public Health Association Annual Meeting; Washington, DC. 2004. [Google Scholar]
- Graves K, Kaskutas LA. Beverage choice among Native American and African American urban women. Alcohol Clin Exp Res. 2002;26:218–222. [PubMed] [Google Scholar]
- Holford NHG. Complex PK/PD Models-an alcoholic experience. Int J Clinical Pharmaco Ther. 1997;35:465–468. [PubMed] [Google Scholar]
- Human Genome Organization Gene Nomenclature Committee. 2001 Available at: http://www.gene.ucl.ac.uk/nomenclature/genefamily/ADH.shtml.
- Jones AW, Hahn RG, Norberg A. Pharmacokinetics of ethanol in plasma and whole blood; Estimation of total body water by the dilution principle. Eur J Clin Pharmacol. 1992;42:445–448. doi: 10.1007/BF00280133. [DOI] [PubMed] [Google Scholar]
- Jones AW, Jonsson KA. Food-induced lowering of blood-ethanol profiles and increased rate of elimination immediately after a meal. J Forensic Sc. 1994;39:1084–109. [PubMed] [Google Scholar]
- Jones AW, Jonsson K-A, Kechagias S. Effect of high-fat, high-protein, and high-carbohydrate meals on the pharmacokinetics of a small dose of alcohol. Br J Clin Pharmacol. 1997;44:521–526. doi: 10.1046/j.1365-2125.1997.t01-1-00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr LA, Greenfield TK. The average ethanol content of beer in U.S. and individual states:Estimates for use in aggregate consumption statistics. J Stud Alcohol. 2003;64:70–74. doi: 10.15288/jsa.2003.64.70. [DOI] [PubMed] [Google Scholar]
- Kolbe L, Kann L, Collins J. Overview of the Youth Risk Behavior Surveillance System. Public Health Reports. 1993;108(Suppl 1):2–10. [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA, Wallace JM. Health risk and inequitable distribution of liquor stores in African American neighborhoods. Soc Sci Med. 2000;51:613–617. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Li T-K, Beard JD, Orr WE, Kwo PY, Ramchandani VA, Thomasson HR. Variation in ethanol pharmacokinetics and perceived ethnic differences. Alcohol Clin Exp Res. 2000;24:415–416. [PubMed] [Google Scholar]
- Li T-K, Yin S-J, Crabb DW, O'Connor S, Ramchandani VA. Genetic and Environmental Influences on Alcohol Metabolism in Humans. Alcohol Clin Exp Res. 2001;25:136–144. [PubMed] [Google Scholar]
- Lundquist F, Wolthers H. The kinetics of alcohol elimination in man. Pharmacol Toxicolo. 1958;14:265–289. doi: 10.1111/j.1600-0773.1958.tb01164.x. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Drinks of the Father: Father’s Maximum Number of Drinks Consumed Predicts Externalizing. Disorders, Substance Use, and Substance Use Disorders in Preadolescent and Adolescent Offspring. 2002;26(12):1823–1832. doi: 10.1097/01.ALC.0000042222.59908.F9. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:135–139. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Williams JD, Qualls WJ. Target marketing of tobacco and alcohol-related products to ethnic minority groups in the United States. Ethn Dis Winter-Spring. 1996;6:83–88. [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, Yesavage JA. Ethanol Pharmacokinetics in White Women: NonLinear Model Fitting Versus Zero-Order Elimination Analyses. Alcohol Clin Exp Res. 2000;24:1353–62. [PubMed] [Google Scholar]
- Neale MC, Martin NG. The effects of age, sex, and genotype on self-reported drunkenness following a challenge dose of alcohol. Behav Genetic. 1989;19:63–78. doi: 10.1007/BF01065884. [DOI] [PubMed] [Google Scholar]
- Pikaar NA, Wedel M, Hermus RJJ. Influence of Several Factors on Blood Alcohol Concentrations after drinking Alcohol. Alcohol & Alcoholism. 1988;23:289–297. [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li T-K, O'Connor S. A physiologically based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without Family histories of Alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addition Research Foundation; 1995. [Google Scholar]
- Sorbel J, Morzorati S, Connor S, Li T-K, Christian JC. Alcohol effects on the heritability of EEG spectral power. Alcohol Clin Exp Res. 1996;20:1523–1527. doi: 10.1111/j.1530-0277.1996.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Thomasson HR, Beard JD, Li TK. ADH2 Gene Polymorphisms are Determinants of Alcohol Pharmacokinetics. Alcohol Clin Exp Res. 1995;19:1494–9. doi: 10.1111/j.1530-0277.1995.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Christian JC, Rose RJ. Genetic analysis of behavioral measures in an alcohol challenge study (abstract) Behav Genet. 1995;25:293. [Google Scholar]
- Wang MQ, Nicholson ME, Jones CS, Fitzhugh EC, Westerfield CR. Acute alcohol intoxication, body composition, and pharmacokinetics. Pharmacol Biochem Behav. 1992;43:641–3. doi: 10.1016/0091-3057(92)90205-t. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. The American Journal of Clinical Nutrition. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Martin NG. Alcohol Consumption and Alcohol Pharmacokinetics: Interactions Within the Normal Population. Alcohol Clin Exp Res. 1994;18:238–43. doi: 10.1111/j.1530-0277.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Carr LG, Bosron WF, Li T-K, Edenberg HJ. Genotyping of human alcohol dehydrogenases at the ADH2 and ADH3 loci following DNA sequence amplification. Genomics. 1988;2:209–214. doi: 10.1016/0888-7543(88)90004-3. [DOI] [PubMed] [Google Scholar]