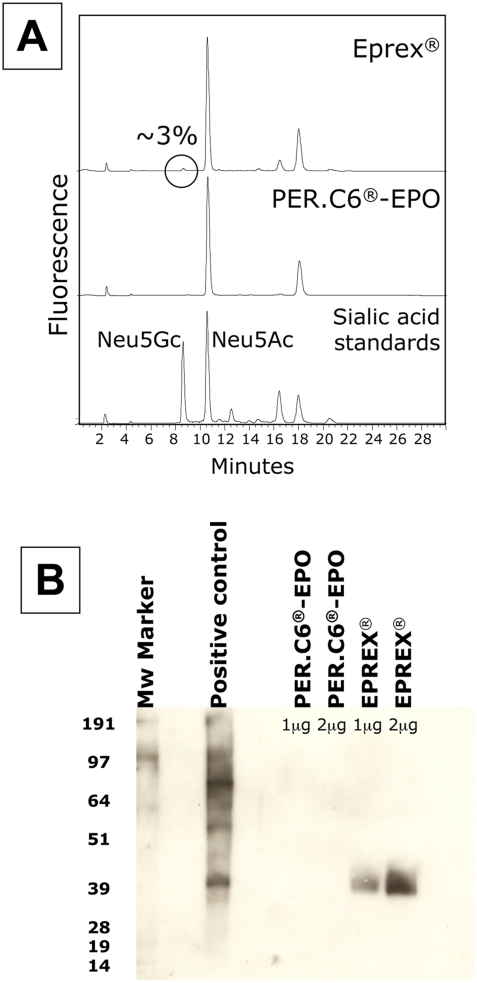
Figure 6. Lack of Neu5Gc in a biotherapeutic agent prepared in the human PER.C6® cell line under Neu5Gc-free/serum-free conditions.
PER.C6® cells were stably transfected with cDNA encoding human EPO and co-transfected with ST3GalIV for full sialylation of the glycans of EPO. EPO-expressing PER.C6® cells were cultured under serum-free conditions (VPRO medium) and EPO was purified from the medium. The sialic acid content of PER.C6®-rEPO and of CHO-rEPO (Eprex) was similar as determined by IEF (data not shown). A. The presence of Neu5Gc on CHO-rEPO (Eprex) or PER.C6®-rEPO was examined by DMB-HPLC according to [38]. B. The presence of Neu5Gc on both CHO-rEPO (Eprex) and PER.C6®-rEPO was examined by the highly sensitive Western blot as described in the Methods. 1 and 2 µg of EPO and 5 µg of positive control (bovine fetuin) were run on a 4–12% SDS-PAGE gel and blotted onto nitrocellulose membrane. Immunostaining for Neu5Gc was performed as described in the Methods and as above under figure 2.