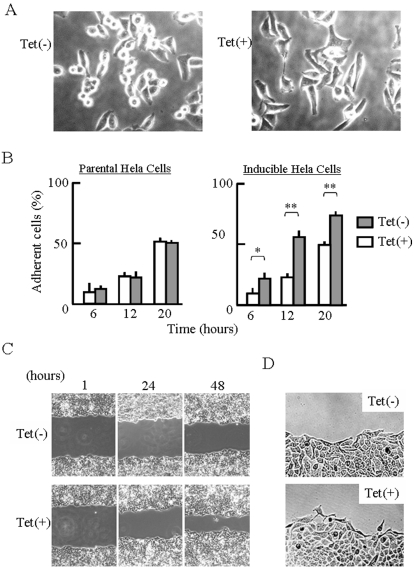
Figure 4. Enhancement of cell spreading and motility by scapinin.
(A) Enhancement of cell spreading by scapinin. Inducible Hela cells were seeded onto an 8-chambered glass-slide and then cultured with (Tet+) or without (Tet−) 0.1 µg/ml tetracycline. Cell morphology was monitored with a light microscope and photographed at 12 hours. (B) Inducible Hela cells were treated as in (A), and cell morphology was monitored at specified times. Adherent and non-adherent cells were counted under a microscope. Data are expressed as mean±SED from four independent experiments. Student's t test: *: P<0.05; **: P<0.01. The parental Hela cells were also cultured with (Tet+) or without (Tet−) of tetracycline, and cell morphology was monitored. (C) Enhancement of cell motility by scapinin. To measure cell motility, we used a wound healing assay. Hela cells were cultured on 6-well plates until confluence. The confluent monolayer cultures were treated with (Tet+) or without (Tet−) 0.1 µg/ml tetracycline for 4 hours and were then wounded with a straight scratch using a yellow pipette tip. After washing them three times with serum-free DMEM, the wounded monolayer cultures were further incubated (Tet+) with or without (Tet−) 0.1 µg/ml tetracycline in DMEM containing 1% fetal bovine serum. To reduce cell growth, the serum concentration was reduced to 1%. At 1, 24, and 48 hours after the wounding, the cell monolayer was photographed. The front of cell migration at 24 hours was shown in (D).