Abstract
Lack of expression of neurofibromin in neurofibromatosis 1 and its lethal derivative, malignant peripheral nerve sheath tumors (MPNSTs), is thought to result in the overactivation of the Ras signaling pathway. Our previous studies have shown that cells with overactivation in the Ras pathway are more permissive to infection with herpes simplex virus 1 and its mutant version R3616. In this study, we show that among five different mouse MPNST cell lines, only the ones with elevated levels of Ras signaling are highly permissive to infection with oncolytic herpes G207. Specific inhibitors of the Ras, ERK, and JNK pathways all reduced the synthesis of viral proteins in MPNST cells. The cell lines that contained lower levels of Ras and decreased activation of downstream signaling components underwent an enhancement in apoptosis upon exposure to G207. Additionally, mouse SW10 Schwann cells were able to become infected by parental herpes but were found to be resistant to G207. The immortalization of these cell lines with the expression of SV40 large T antigen increased the levels of Ras activation and permissiveness to oncolytic herpes. A Ras/Raf kinase inhibitor reduced the synthesis of both herpes simplex virus-1 and G207 proteins in SW10 cells. The results of this study, therefore, introduce Ras signaling as a divergent turning point for the response of MPNST cells to an assault by oncolytic herpes.
The Ras signal transduction pathway acts as a central hub through which a variety of cell functions are controlled. The influence of Ras signaling on the responsiveness of cancer cells to therapy makes this complicated molecular machinery a critical area of inquiry in basic and translational research.1,2,3,4 Overactivation of the Ras proto-oncogene occurs in more than 30% of human malignancies,5 whereas activation of its downstream elements triggers pro-oncogenic events leading to neoplasia and malignancy.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 Ras activation is regulated by the interplay between two groups of proteins: Ras-guanine nucleotide exchange factors (GEFs), which activate Ras by exchanging guanosine diphosphate (GDP) to guanosine triphosphate (GTP), and Ras-GTPase activating proteins (Ras-GAPs), which function by the process of conversion of Ras-GTP (active) to Ras-GDP (inactive).21 Neurofibromin, a Ras-GAP, negatively regulates Ras output by accelerating the hydrolysis of Ras-GTP to Ras-GDP.22 Mutations in the neurofibromin coding gene (nf1) cause neurofibromatosis type 1 (NF1), an autosomal-dominant human genetic disease with an incidence of ∼1 in 2500 births.21,23,24 Malignant peripheral nerve sheath tumors (MPNSTs) arise from plexiform neurofibromas in 10 to 15% of NF1 patients. MPNST is considered the main cause of mortality in adult NF1 patients, with only 34 to 52% of patients surviving for 5 years.25 The molecular events involved in the malignant transformation of benign neurofibromas to MPNST are poorly defined. It has been postulated that one of the main tumor-causing effects of mutations in the tumor suppressor nf1 gene is the subsequent activation of Ras.26,27,28,29,30
Inactivation of both copies of the nf1 gene has been demonstrated in benign human neurofibromas and has been shown to cause tumors in murine models.31 Loss of heterozygosity of nf1 and p53 has frequently been observed in human MPNST.32,33,34 Recombinant mouse strains (NP mice), which harbor inactivated nf1 and p53 alleles (cis-nf1+/−:p53+/−) demonstrate the cumulative effects of loss of both nf1 and p53 genes in the etiology of MPNST.35,36 To understand the extent of the influence of nf1-mutations on the outcome of Ras signaling, we have examined the activation of this pathway in cells isolated from distinct MPNST tumors of NP mice35 that exhibit loss of heterozygosity for both nf1 and p53 genes but no mutations in Ras gene. The results of our study show that although all of the studied MPNST cell lines originated from a cis-nf1+/−:p53+/− background, overactivity in Ras signaling was only observed in two of the five cell lines. Activation patterns of downstream Ras-signaling pathways follow the overactivity seen for Ras: cell lines with higher Ras-GTP levels exhibit elevated levels of kinase activity for extracellular signal-related kinase (ERK), Jun amino-terminal kinase (JNK), p38-kinase, and phosphatidylinositol 3-kinase (PI3K). In the next step, we linked the overall Ras-signaling portfolio of MPNST cells to biological characteristics that may influence the outcome of therapy. The apoptotic capabilities of MPNST cells and their permissiveness to G207, an oncolytic version of herpes simplex virus-1 (HSV-1),37,38 was studied. G207 has been explored for its therapeutic efficacy in a murine model of MPNST.39
Upon infection with G207, MPNST cells with increased Ras signaling exhibited higher viral permissiveness than cell lines with lower levels of Ras signaling. The cells with lower Ras signaling underwent apoptosis after exposure to G207. This finding is in agreement with our previous studies in which Ras signaling was correlated to the permissiveness of cells to herpesvirus40,41 and other viruses such as reovirus.42,43,44 For MPNST cells with both high- and low-Ras signaling, a significant decrease in proliferation and invasiveness was observed. Further, we have observed that untransformed Schwann cells (with low-Ras activation) remain nonpermissive to G207 infection. However, these cells become more permissive to infection upon conditional immortalization (by expression of SV40 large T antigen), which induces the Ras-Raf pathway. Blockers of Raf, Ras, ERK, and JNK all inhibit the production of viral proteins. The results of this study, therefore, not only expand our fundamental understanding about the regulation of Ras signaling in MPNST cells but also correlate such information with the responsiveness of MPNST tumor cells to oncolytic HSV as a model for linking signaling characteristics and therapeutic outcomes.
Materials and Methods
Cell Culture and Viruses
Mouse MPNST cells (6IE4, 37-3-18, 35-1-2, 38-2-18, and 32-5-24-12 cells) were originally isolated from different mouse MPNSTs.35 Mouse Schwann cells, SW10, were purchased from American Type Culture Collection, Rockville, MD (American Type Culture Collection number CRL-2766). All cells were maintained in Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, and additional antibiotics. G207 was prepared as described previously37 or provided by MediGene, Inc., San Diego, CA. HSV-1(F) virus was a gift from Dr. Bernard Roizman (University of Chicago, Chicago, IL).
Affinity Pull-Down Assays for Ras
Ras activation assay were performed in accordance to the manufacturer’s (Upstate, Lake Placid, NY) instruction. Briefly, cells grown in 10-cm tissue culture dishes were lysed at 75 to 80% confluency in magnesium lysis buffer. After determination of protein concentration, 10 μl of Ras assay reagent (Raf-1 binding domain-agarose) was added to 200 μg of total cell protein in 200 μl of magnesium lysis buffer. After a period of rocking at 4°C, the activated (GTP) form of Ras bound to the agarose beads was collected by centrifugation, washed, boiled in 2× Laemmli reducing sample buffer (Bio-Rad, Hercules, CA) and loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Bio-Rad). The proteins were transferred to a nitrocellulose membrane; blocked with Tris-buffered saline/0.2%Tween/5% milk (TBST-MLK) and incubated with Pan-Ras antibody (1:1000). The membrane was then washed with TBST for 10 minutes each and then incubated with sheep anti-mouse horseradish peroxidase-conjugated IgG (1:2000; Amersham, Arlington Heights, IL). Bands were detected using LumiGLO chemiluminescent reagent peroxide (Cell Signaling, Beverly, MA).
Nonradioactive Ras Downstream Kinase Assays
Nonradioactive Ras effector assays were performed in accordance with the manufacturer’s (Cell Signaling) instructions. Briefly, cells grown in 10-cm tissue culture dishes were lysed at 75 to 80% confluency with 300 μl of cell lysis buffer. Immobilized antibody-bead slurry capable of binding to the phosphorylated forms of ERK, JUN, AKT or p38-kinase was then added to 200 μg of total cell protein in 200 μl of cell lysis buffer. The mixture was incubated with gentle rocking overnight at 4°C then collected, washed, and introduced to an in vitro kinase reaction in presence of ATP and a special buffer. The levels of phosphorylated ELK, JUN, ATF2, and AKT were then assayed by Western blotting using antibodies directed against phosphorylated forms of these proteins.
Early (Annexin-V Assay) and Late Phase [Terminal dUTP Nick-End Labeling (TUNEL) Assay] for Detection of Apoptosis
Annexin-V labeling was performed according to the manufacturer’s (Calbiochem, La Jolla, CA) recommendations for the conventional Annexin-V binding protocol. Briefly, cells were washed in phosphate-buffered saline (PBS) with gentle centrifuge (1000 × g for 5 minutes at room temperature) and resuspended in 0.5 ml of cold 1× binding buffer with the addition of 1.25 μl of Annexin V-fluorescein isothiocyanate (FITC). After incubation for 15 minutes, cells were centrifuged, supernatant was removed, and cells were resuspended in 0.5 ml of cold 1× binding buffer. In the next step, 10 μl of propidium iodide were added and samples were placed on ice in the dark and then analyzed by a flow cytometer using an argon 488-nm argon ion laser source. TUNEL assay was also performed according to the recommendations by the manufacturer (Chemicon, Temecula, CA). Briefly, cells were fixed in paraformaldehyde and alcohol and then incubated at −20°C overnight. After rehydration of cells in Tris-buffered saline, they were permeabilized by proteinase K, equilibrated by terminal deoxynucleotidyl transferase (TdT) buffer, and labeled by TdT reaction mixture/enzyme at 37°C for 1.5 hours in the dark. The reaction was then stopped by washing with Tris-buffered saline and cells were analyzed by a flow cytometer using an argon 488-nm argon ion laser source.
Immunofluorescent Analysis of HSV-1 Infection
MPNST and Schwann cells were grown in eight-well slide chambers (Falcon, San Jose, CA) and infected with HSV-1 (strain F) or G207 or mock-infected. At different times after infection, cells were fixed in acetone (100%) for 10 minutes and then left at room temperature to dry before incubation with a fluorescein-labeled mouse monoclonal antibody against HSV-1 gC antigen (Labvision, Fremont, CA) for 30 minutes at 37°C. The slides were washed with distilled water, dried, and mounted in 90% glycerol containing 0.1% phenylenediamine, and viewed with an Axiophot microscope (Carl Zeiss, Thornwood, NY) on which a Carl Zeiss camera was mounted. Pictures were captured by an attached computer and processed with appropriate software.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blot Analysis
Different cells were lysed with a single detergent lysis buffer [50 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, 0.02% sodium azide, 100 μg/ml phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin, and 1% Triton X-100], normalized for the amount of total protein and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a Bio-Rad minicell protein-II system (using precast 10% discontinuous gels) followed by electroblotting onto nitrocellulose paper. The membrane was then washed and incubated with a primary rabbit antibody against all HSV-1 antigens (DAKO, Carpinteria, CA), followed by the horseradish peroxidase-conjugated secondary antibody. After extensive washing, the blot was exposed to Lumigel (Viagen, Tempa, FL) detection solution and subjected to autoradiography.
Exposure of Cells to the Inhibitors of Cell Signaling
Uninfected 37-3-18 cells were exposed to Ras and its downstream specific inhibitors overnight at the following concentrations: AG-14178 at 0.5 μmol/L, L-744,832 at 60 μmol/L, PD98059 at 20 μmol/L, and SP600125 at 25 μmol/L. Schwann cells were exposed to Raf1 kinase inhibitor-II. L-744,832 and Raf1 kinase inhibitor-II were obtained from Calbiochem. AG14178, PD98059, and SP600125 were obtained from Biosource (Camarillo, CA).
Plaque (Viral Progeny) Titration Assay
Plaque titration was performed with the purpose of evaluating the yield of progeny virus at 24 hours after infection. Briefly, the supernatant from infected cells were serially diluted from 1/10 to 1/108. A volume of 300 μl from each dilution was added to duplicate wells (of six-well plates) containing Vero cells at ∼75% confluency after removal of the existing media and rinsing the cells with PBS. Cells were then incubated at 37°C until development of cytopathic effects in the form of plaques, which usually occurs within 2 to 3 days. At this point, a monolayer was fixed with methanol and stained with Giemsa solution for 10 minutes. The number of clear plaques was then determined by calculating the average number of plaques per well for each dilution and the volume used to infect each well.
Cell Invasion Assay
To evaluate cell invasiveness, a commercial kit was used (BD Biosciences, San Jose, CA). Briefly, cells (50,000 control or test cells) were introduced into Matrigel-coated inserts fitting 24-well plates. As cells invaded through the layer of Matrigel, the fraction of invaded cells were detected by staining with crystal violet and quantifying by spectroscopy. Invaded cells were fixed with 5% paraformaldehyde and stained with a 5% solution of crystal violet and then photographed to obtain a visual representation of their density. The cells were then solubilized in a 3% detergent (Nonidet P-40) solution, and the absorbance was measured by spectrophotometry at 590 nm.
Cell Proliferation Assay
The cell proliferation assay was performed using a kit (Chemicon) according to the manufacturer’s instructions. The assay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. Expansion in the number of viable cells results in an increase in the overall activity of the mitochondrial dehydrogenases resulting in an increase in the amount of formazan dye formed. Briefly, 104 cells per well were seeded in a 96-well microplate in volume of 100 μl per well. At different times, 10 μl of WST-1/ECS solution was added to each well. The plates were incubated for 4 hours in standard culture conditions. The plates were then shaken thoroughly for 1 minute and absorbance was measured at 480 nm.
Statistical Analysis
Results are reported as means ± SD. Student’s t-test was used to analyze statistical differences between groups. α level was set at 0.05.
Results
MPNST Cell Lines Vary in Their Levels of Ras and ERK Pathway Activation
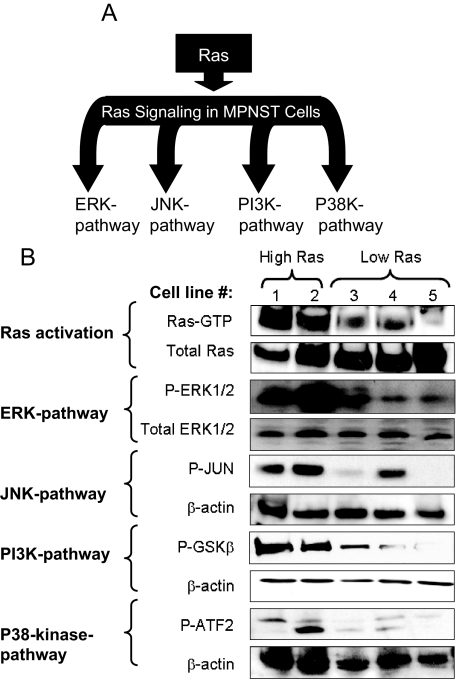
Ras activation stimulates a variety of effector pathways including ERK, JNK, p38 kinase, and PI3K (Figure 1A).1 Because the Ras-GAP function of neurofibromin is lost in the MPNST cell lines used in this study, we were interested in elucidating the effects of such Ras-deregulating genetic abnormalities on Ras activation and signaling. To achieve this, the amount of Ras-GTP was determined using an affinity precipitation assay effectively differentiating between active and inactive forms of Ras. As is shown in Figure 1B, among the five studied mouse MPNST cell lines, only 6IE4 and 37-3-18 showed elevated levels of Ras-GTP as compared with the other three MPNST cell lines (35-1-2, 38-2-18, and 32-5-24-12). The activated levels of Ras (H-, K-, and N-Ras) were determined under growth conditions in the presence of 10% fetal bovine serum. We also evaluated the amount of the active (phosphorylated) form of ERK (P-ERK) and found that at equal total enzyme levels, P-ERK levels were higher in 6IE4 and 37-3-18 as compared with the other three MPNST cell lines.
Figure 1.
Activity of Ras and its downstream signaling pathways in mouse MPNST cells. A: Schematic representation of Ras and its downstream effector pathways. B: The activity of Ras in different mouse MPNST cell lines was detected by Ras affinity pull-down assay for Ras-GTP. The first panel shows the amount of activated form of Ras (Ras-GTP), and the second panel shows the amount of total form of Ras. Western blotting using phospho-specific antibody against ERK1/2 was used to detect the amount of activated ERK in MPNST cells in comparison to the amount of total form of this enzyme. The activities of other Ras downstream effector pathways were investigated by pull-down assays coupled with in vitro kinase reactions for JUN, P38, and PI3K pathways. In each assay an in vitro kinase reaction was performed to detect the capability of each effector in phosphorylating its specific substrate, which was in turn detected by blotting with a phospho-specific antibody. Cell lines are numbered as follows: lane 1, 6IE4; lane 2, 37-3-18; lane 3, 35-1-2; lane 4, 38-2-18; lane 5, 32-5-24-12.
MPNST Cells Exhibit Variable Levels of Stress-Activated MAPK and PI3K Pathway Activity
JNK and p38-kinase pathways are known as stress-activated MAPK pathways.45 We evaluated the amount of the phosphorylated forms of the JNK substrate, JUN, and the p38-kinase substrate, ATF-2, in lysates of MPNST cells after in vitro kinase reactions. As shown in Figure 1B, increased activities for both pathways were detected in 6IE4 and 37-3-18 as compared to other MPNST cell lines in the absence of external stress stimuli. Notably, the cell line 38-2-18 also showed elevated levels of JNK pathway activity, independent of elevated Ras-GTP.
The PI3K pathway is a downstream effector of Ras with significant anti-apoptotic effects that can greatly influence cell survival.46 We determined the level of PI3K activity in MPNST cell lines by measuring the amount of GSK3α/β phosphorylated on serines 9 or 21 by the phosphorylated form of AKT, a downstream substrate in the PI3K pathway. Once again, the highest levels of activity were observed for cell lines 6IE4 and 37-3-18 cells, as compared to the other cell lines.
Ras Signaling in MPNST Cells Increases Resistance to Apoptosis
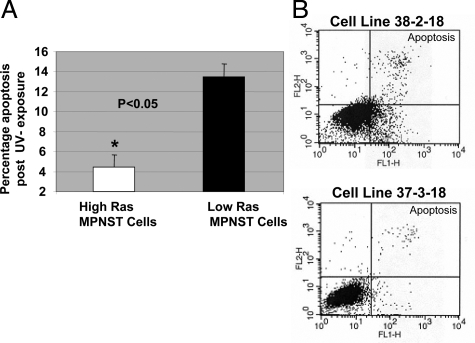
Increased levels of Ras signaling can enhance cell resistance to apoptosis by activation of the PI3K pathway and its multiple effectors47 such as 3-phosphoinositide-dependent protein kinase 1 (PDK1) and protein kinase-B (PKB/AKT).48 Therefore, MPNST cells with higher levels of Ras-GTP and AKT activation may be characterized by increased resistance to pro-apoptotic signals. To investigate this, we exposed MPNST cells to UV irradiation (10 mJ/cm2), which triggers p53-independent apoptosis.49,50 Early induction of apoptosis was evaluated by performing the Annexin-V assay. The percentage (mean ± SD) of apoptotic 6IE4 and 37-3-18 cells (high-Ras group) was approximately three times lower than the other three MPNST cell lines with low-Ras-GTP and AKT activity levels (∼4.5% versus ∼13%; Figure 2A). Figure 2B shows a representation of the Annexin-V labeling for apoptotic cells obtained for cell lines 38-2-18 and 37-3-18. The basal level of apoptosis in the absence of any controllable insult was found to be ∼2% in multiple experiments for all MPNST cell lines (data not shown).
Figure 2.
Induction of apoptosis after UV exposure in MPNST cells. MPNST cells with high- and low-Ras signaling activity were exposed to UV (10 mJ/cm2) and the induction of apoptosis was measured after 6 hours using the Annexin-V conversion test. A: The average (±SD) percentage of apoptosis occurring after UV exposure is plotted for two groups including high-Ras (6IE4 and 37-3-18) and low-Ras MPNST cells (35-1-2, 38-2-18, and 35-5-24-12). B: Annexin-V labeling results obtained for 37-3-18 and 38-2-18 cells after exposure to UV. The top right quadrant represents cells committed to undergoing apoptosis.
MPNST Cells with Higher Levels of Ras Signaling Show Increased Permissiveness to G207 Herpesvirus
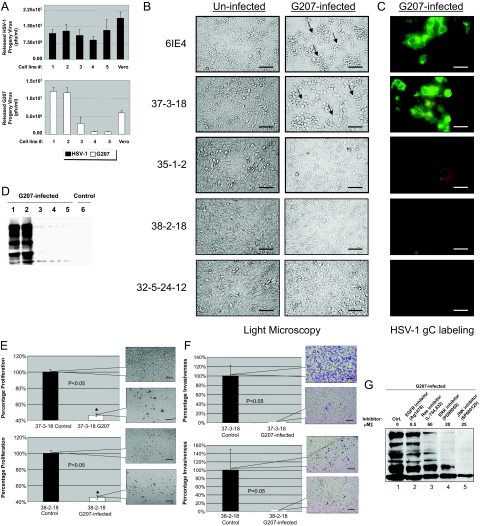
G207 is a recombinant oncolytic HSV-1 that has a deletion in both copies of the viral γ134.5 gene and interruption of the viral UL39 gene coding for ICP6, the large subunit of HSV ribonucleotide reductase.37 MPNST cells were infected with G207 at a multiplicity of infection (MOI) of 2 (pfu/cell). In a single-step growth experiment, infection with G207 yielded a higher titration of progeny virus in cells with higher levels of Ras signaling (6IE4 and 37-3-18) as compared with 35-1-2, 38-2-18, and 32-5-24-12 cells. The parental wild-type HSV-1 strain F, however, generated a comparable amount of progeny virus in all cell lines (Figure 3A). Similarly, cytopathic effects (rounding and detachment of cells) were predominantly observed in 6IE4 and 37-3-18 at 24 hours after infection (Figure 3B). Expression of glycoprotein-C (gC), a late envelope protein used frequently for detection of herpetic infection, was also higher in infected 6IE4 and 37-3-18 cells than 35-1-2, 38-2-18, and 32-5-24-12 cells (Figure 3C). Western blot analysis of G207-infected cells demonstrated higher levels of HSV-1 proteins in high-Ras-signaling MPNST cell lines (Figure 3D). No proteins were detected in lysates from uninfected 6IE4 cells (Figure 3D, lane 6). These findings show that cells with higher levels of Ras signaling (6IE4 and 37-3-18) are more permissive to infection with G207 virus.
Figure 3.
Permissiveness of MPNST cells to infection by HSV-1(F) and G207. A: The released progeny virus was titrated after exposure of different MPNST cell lines as well as Vero cells to G207 (an oncolytic version of HSV-1, white columns) or the parental virus, HSV-1, strain F (black columns), at the 20th hour after infection at MOI ∼ 2. The number of plaques/ml (as a representation of infectious viral particles) was counted after staining with Giemsa in independent experiments. B: Morphology of MPNST cells lines in the presence and absence of G207 (MOI ∼ 2) virus for 24 hours as studied by light microscopy (arrows point to monolayer showing rounding and clumping typical of herpetic infection). C: Expression of HSV-1 glycoprotein-C (gC) in G207-infected MPNST cells. A FITC-conjugated mouse monoclonal antibody was used to detect the expression of the HSV-1 glycoprotein C (gC) in different MPNST cell lines 24 hours after infection with G207. The green color represents expression of this viral envelope protein. D: Western blot analysis of HSV-1 proteins produced in G207-infected MPNST cells. The viral protein production was evaluated in five MPNST cells after 24 hours of infection with G207 using a polyclonal antibody raised against HSV-1 antigens. Control 6IE4 cells (lane 6) were not infected and served as an indicator for the specificity of the antibody. E: Proliferation of 37-3-18 and 38-2-18 cells after infection with G207 (MOI ∼ 1) was compared to uninfected control cells at 48 hours after infection (control cells are plotted as 100%). Callout panels show the density of cells at this time point. F: Invasiveness of 37-3-18 cells and 38-2-18 cells after infection with G207 (MOI ∼ 1) was compared to uninfected control cells at 48 hours after infection (control cells are plotted as 100%). Notably, control 38-2-18 cells were much less invasive than control 37-3-18 cells. Callout panels show the density of invaded cells at this time point. G: Effects of Ras signaling inhibitors on production of G207 proteins in the 37-3-18 cell line. One of the most permissive MPNST cell lines, 37-3-18, was exposed to different inhibitors of Ras signal transduction pathway overnight and then infected with the G207. Cells were lysed after 24 hours after infection and the amount of viral proteins in the lysates was detected by Western blotting. Inhibitors were used at the following concentrations: AG1478 (an EGFR blocker) at 0.5 μmol/L, L-744,832 (a Ras farnesylation blocker) at 60 μmol/L, PD98059 (an ERK pathway blocker) at 20 μmol/L, and SP600125 (a JNK-pathway blocker) at 25 μmol/L. Cell lines are numbered as follows: lane 1, 6IE4; lane 2, 37-3-18; lane 3, 35-1-2; lane 4, 38-2-18; lane 5, 32-5-24-12. Scale bars: 600 μm (B, E, F); 300 μm (C).
Viral Replication Reduces Proliferation and Invasiveness of MPNST Cells
Once MPNST cells were infected with G207, it was important to investigate the impact of infection on cell proliferation and invasiveness. Cells with both high (37-3-18) and low (38-2-18) levels of Ras signaling exhibited a significant (P < 0.05) loss of viability (Figure 3E) and invasiveness (Figure 3F) upon exposure to G207. Callout panels show the density and morphology of these cells at 48 hours after infection. Notably, control 38-2-18 cells were less invasive than 37-3-18 cells (Figure 3F).
Viral Protein Synthesis Is Reduced by Ras Signaling Inhibitors in MPNST Cells
Ras signaling can be efficiently repressed when plasma membrane association of Ras, phosphorylation of ERK1/2 (by MEK1/2), or activation of JNK1/2 is blocked by their specific inhibitors. As shown in Figure 3G, the synthesis of viral proteins is decreased when 37-3-18 cells are exposed to effective and nontoxic concentrations of these inhibitors. The blockade of Ras farnesylation (by the FTI compound L-744,832 at 60 μmol/L) or its downstream pathways such as ERK (by PD90859 at 20 μmol/L) and JNK (by SB600125 at 25 μmol/L) reduced the level of viral protein synthesis. However, a specific blocker of epidermal growth factor receptor (EGFR), AG14718 (at 0.5 μmol/L), was unable to cause any prominent difference in this regard.
Infection with G207 Induces Apoptosis in MPNST Cells with Lower Levels of Ras Signaling
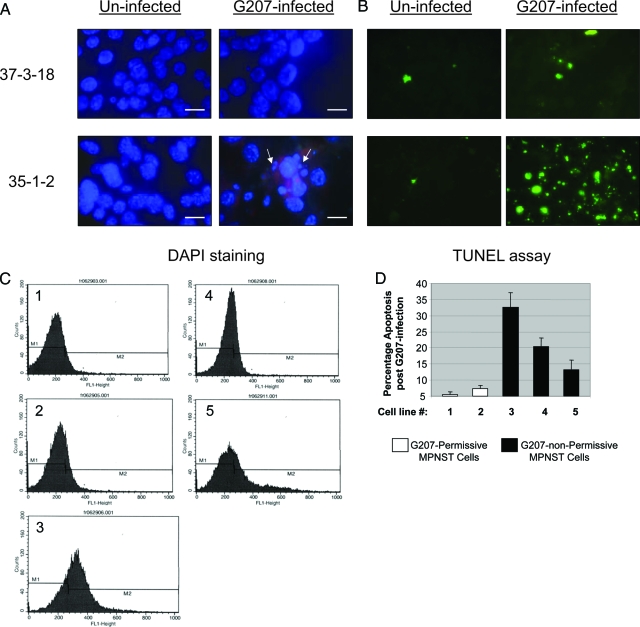
Oncolytic viruses are designed to cause tumor regression by inducing a cascade of lytic infection. G207 is capable of inducing such lytic infection in MPNST cell lines with higher Ras signaling (Figure 3). MPNST cells with low levels of Ras signaling, however, are poorly permissive to infection with G207 at 24 hours after exposure to this virus (Figure 3, A–C). Nuclear 4′,6-diamidino-2-phenylindole-staining (DAPI) of G207-infected MPNST cells with low-Ras signaling revealed patterns of chromatin condensation reminiscent of apoptosis, as exemplified by results for 35-1-2 cells (Figure 4A). In contrast, the majority of nuclei of MPNST cells with high-Ras signaling did not demonstrate such fragmentation on exposure to G207, as shown for 37-3-18 cells in Figure 4A.
Figure 4.
Induction of apoptosis after infection of MPNST cells with G207. A: Nuclear morphology of infected cells was studied by 4′,6-diamidino-2-phenylindole (DAPI) staining (the red background in bottom right panel is because of the Texas Red component of anti-gC antibody preparation) (arrows show nuclei with chromatin condensation). B: Different MPNST cell lines were infected with G207 (MOI ∼ 2). Twenty-four hours after infection the occurrence of apoptosis was evaluated using TUNEL assay. The cells were fixed on a slide and studied with a fluorescence microscope for FITC labeling of nucleosome-sized DNA. C: The panels show fluorescence-activated cell sorting data as the counts of sorted cells (fluorescent events) versus the intensity of FITC used for labeling the nucleosome-sized DNA in MPNST cells undergoing apoptosis after 24 hour of infection with G207. D: The graph shows the percentage (mean ± SD) of apoptotic cells for each MPNST cell line after 24 hour of exposure to G207. Cell lines are numbered as follows: lane 1, 6IE4; lane 2, 37-3-18; lane 3, 35-1-2; lane 4, 38-2-18; lane 5, 32-5-24-12. Scale bars = 300 μm.
To further verify the occurrence of apoptosis, a TUNEL assay was performed 24 hours after treating MPNST cells with G207 (MOI∼2). Apoptosis was prominently observed in MPNST cells with low-Ras signaling as the result of infection with G207 (Figure 4B, results shown for 35-1-2 cells). Figure 4C shows the results of fluorescence-activated cell sorting analysis on MPNST cells labeled by TUNEL assay at 24 hours after infection with G207 (cells were fixed on a slide and studied with a fluorescent microscope). When these cells were quantified by fluorescence-activated cell sorting, the G207 nonpermissive cells (35-1-2, 38-2-18, and 32-5-24-12) exhibited higher levels of apoptosis compared to G207-permissive cells (6IE4 and 37-3-18; Figure 4D).
Immortalization Increases the Permissiveness of Schwann Cells to G207 While Enhancing the Level of Ras-GTP
Schwann cells undergo malignant transformation upon loss of the nf1 and p53 genes in mice and humans, resulting in the development of MPNST.35,36,51 Because Schwann cells are considered as nonmalignant counterparts of MPNST, we were interested to investigate the permissiveness of these cells to G207. This would be not only of interest from the biological point of view (to test the capabilities of G207 in infecting Schwann cells with low levels of Ras activation) but is also important from a clinical point of view to understand the specificity of this virus against tumor cells. To achieve this we used SW10 mouse Schwann cells, generated by infecting primary Schwann cells with a retroviral vector encoding a temperature-sensitive SV40 large T-antigen (SV40-LT) transgene.52 Such cells express SV40-LT at 33°C (proliferative phenotype), but do not express this protein at 37°C (nonproliferative phenotype). Immortalization induced by SV40-LT involves interference with the tumor suppressors p53 and pRB,53 and is dependent on Ras signaling.54 SV40-LT expression also induces Raf.55
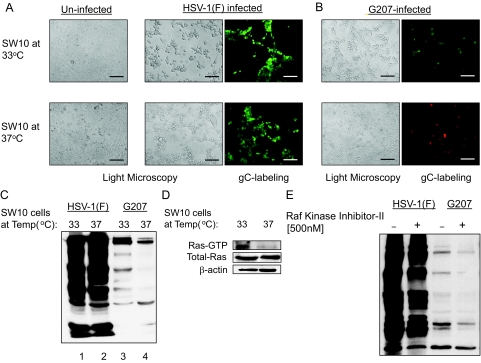
As shown in Figure 5A, SW10 cells cultured at 33°C and 37°C appeared comparably permissive to wild-type HSV-1 (strain F) replication, whereas G207 mainly replicated in SW10 cells at the proliferative temperature (Figure 5B). A comparable amount of HSV-1 protein synthesis was seen at both conditions for HSV-1, as determined by Western blot analysis (Figure 5C). In the case of G207, however, viral protein expression mainly occurred in proliferating cells. Therefore, cellular conditions supporting immortalization by SV40-LT enhance the permissiveness of these cells to infection by the mutant virus G207, whereas the parental HSV-1(F) virus infects Schwann cells with comparable efficacy in both immortalized and nonimmortalized conditions. It has been proposed that Ras signaling is required for immortalization of cells by SV40-LT.54 Therefore, we evaluated the levels of Ras-GTP in SW10 cells at 33°C and 37°C. As shown in Figure 5D, an increase in the Ras-GTP fraction was observed in Schwann cells cultured at 33°C, which may explain the increased permissiveness to G207 infection at this temperature. In addition to Ras, Raf-kinase may also be activated by SV40-LT.54,55We investigated whether inhibiting this pathway in SV40-LT immortalized cells might influence their permissiveness to HSV-1 and G207. In the presence of the Raf-kinase inhibitor-II (500 nmol/L), the amount of herpetic proteins synthesized in immortalized SW10 cells was slightly decreased for both G207 and wild-type virus (Figure 5E).
Figure 5.
Permissiveness of Schwann cells to HSV-1(F) and G207. A: SW10 mouse Schwann cells were incubated at 33°C (proliferative because of SV40-LT expression) and 37°C (nonproliferative) and infected with HSV-1(F) and G207 (MOI ∼ 2). At 24 hours after infection with HSV-1(F), cells were studied with light microscopy for appearance of morphology of infection (middle) as well as stained with a FITC-conjugated mouse monoclonal antibody against expression of the HSV-1 glycoprotein C (gC, green spots) as a marker for herpetic infection (right). Morphological changes after infection can be compared with uninfected cells shown in the left panels. B: SW10 cells cultured at different temperatures are infected with G207 virus and the morphological changes (left) as well as expression of gC (right, green spots) were detected by microscopy. The red background staining in bottom right panel is because of the Texas Red component of the antibody preparation that stains all cells. This panel is photographed at Texas Red channel to visualize the monolayer confluency. C: The amount of viral protein synthesis in SW10 cells for each virus in different temperature conditions (proliferative because of SV40-LT expression at 33°C and nonproliferative at 37°C) was evaluated by Western blotting for herpes proteins. D: Using affinity pull-down assays, the amount of Ras-GTP (active form of Ras) in SW10 cells at proliferative (33°C) or nonproliferative (37°C) temperatures was evaluated. E: The effects of treatment with a specific Raf-kinase inhibitor (500 nmol/L) on viral protein synthesis in SW10 cells infected with HSV-1(F) and G207 at 33°C was studied by Western blotting for herpes proteins. Scale bars = 600 μm.
Discussion
Ras signaling exerts a myriad of proneoplastic effects via various downstream effectors. Downstream of Ras, the family of mitogen-activated protein kinases (MAPKs) regulates different biological functions such as proliferation, differentiation, and apoptosis.1,56,57,58 The ERK pathway is important because of its contribution to malignant transformation. It is initiated by the activation of Raf by Ras, which eventually results in the activation of the serine/threonine kinases ERK1/2 (p42/44). The ERK pathway is involved in cell-cycle progression and differentiation whereas pathways such as JNK and p38-kinase contribute to stress signal responses and induce differentiation and growth inhibition.59,60,61,62 Although activation of such mechanisms generates a strong growth stimulatory signal for the cell, Ras signaling also down-regulates the apoptotic capabilities via the PI3K-pathway.46,63 Apoptotic inhibition is achieved by AKT-mediated phosphorylation of targets such as BAD, Forkhead transcription factors, and caspase-9.56,64
In NF1, loss of neurofibromin tumor suppressor gene, which encodes a Ras-GAP, lowers the threshold for tumorigenesis. The subsequent deregulation in Ras-GAP activity has been assumed to cause overactivation of Ras activity and its downstream signaling in neurofibromas.26,65,66 Our present data put this assumption into a new perspective by showing that mouse MPNST cells bearing deletions in both copies of the nf1 gene exhibit variations in their Ras-signaling portfolio, resulting in a drastic difference in their response to some therapeutic agents. Two of the five different nf1−/−/p53−/− MPNST cell lines expressed higher levels of Ras-GTP, whereas the other three remained at lower levels.
Oncolytic viruses are novel therapeutic agents with the potential to target cancer cells in a specific manner.67,68 Mutant versions of herpes simplex virus-1 (HSV-1) have been examined in a variety of in vitro and in vivo models as well as phase I clinical trials for their anti-tumor action and safety.38,39,69,70,71,72 We have previously studied the dependence of some of these viruses on signaling pathways such as Ras and PKR.40,41,73 However, further studies are necessary to clarify the precise underlying mechanisms. In this study, we show that the observed variability in Ras signaling in MPNST cell lines influences their biological responses including their behavior against G207. Indeed, MPNST cells with high-Ras-signaling levels exhibited reduced apoptotic capabilities compared to cells with low-Ras signaling, which is consistent with our data on the activity of the PI3K pathway. A higher level of Ras signaling triggers higher PI3K pathway activity as shown by the higher activity of AKT. This in turn inhibits apoptosis with greater efficiency as compared with low-Ras MPNST cells. These results suggest that a substantial variability in therapeutic effects might be observed when an apoptosis-inducing modality is used against different cases of an identical tumor type. On the other hand, blockade of apoptosis is important for supporting effective viral replication because the first cycle of herpes replication happens in 18 to 24 hours after infection, a period in which apoptosis can significantly diminish viral growth.
We observed that MPNST cell lines with elevated levels of Ras signaling appear to be more permissive to G207. In contrast, parental HSV-1 (strain F) was less dependent on Ras signaling and infected all MPNST cells with comparable efficiency in this model. These results are in agreement with our previous studies in which we identified the impairment of dsRNA-activated protein kinase (PKR), the main host cell defense mechanism against viruses, in Ras-transformed cells as the basis for increased permissiveness.41 The viral ICP34.5 (γ134.5 gene product) functions as a viral anti-PKR protein and is capable of increasing dephosphorylation of eukaryotic initiation factor 2α (eIF2α, a downstream molecule in PKR pathway), effectively disabling the anti-viral action of PKR. Therefore, G207 with deletions in both copies of γ134.5 relies on the anti-PKR effects through active Ras signaling for successful replication. The parental HSV-1, however, is capable of exerting some anti-PKR effects because of the existence of γ134.5 and therefore is less dependent for replication on the anti-PKR effects of the Ras pathway. Consequently G207 only replicates efficiently in high-Ras MPNST cells, whereas wild-type HSV-1 is capable of replicating in all five tested cell lines.
If overactivity of Ras signaling increases permissiveness of MPNST cells to G207, specific inhibitors of Ras and its downstream signaling elements should be able to decrease this permissiveness. Indeed, specific inhibition of these pathways resulted in a clear reduction in viral protein synthesis. No effects were observed for the EGFR blocker AG14718, emphasizing the role of other mechanisms for Ras activation. In the MPNST cell lines in which the NF1 mutation causes an overactivation in Ras activity, a decrease in the sensitivity of Ras to upstream elements such as EGFR might occur because of the increased amounts of Ras-GTP. In other words, the lack of neurofibromin provides an oversupply of Ras-GTP because of a decrease in Ras-GTPase activity. This might make the conversion of Ras-GDP to Ras-GTP less dependent on upstream signals such as stimuli from EGFR. This is also supported by the observation that serum starvation does not significantly change the amount of Ras-GTP in high-Ras-GTP MPNST cells (data not shown). Although loss of proliferation in high-Ras cells is mostly because of the lytic infection and therefore cell death via necrosis,74 G207 appears to induce apoptosis in low-Ras MPNST cells suggesting localized cytotoxic effects of this virus. However, virus-induced apoptosis may also reduce viral replication and spread, thus limiting tumor cell killing. On the other hand, the restriction of the area that undergoes lytic infection (because of occurrence of apoptosis at its borders with low-Ras cells) may act as an additional mechanism for anti-tumoral specificity.
We were also interested in evaluating the status of Ras activation in Schwann cells because these cells are considered to be the nontransformed counterpart of MPNST. We found that the levels of Ras-GTP were lower in untransformed SW10 Schwann cells as compared to high-Ras-GTP MPNST cell lines. Ras-GTP levels were increased upon immortalization of these cells by SV40-LT expression. When infected by wild-type HSV-1(F), Ras activation (induced by SV40-LT expression) did not considerably affect permissiveness of SW10 Schwann cells. However, these cells became permissive to G207 only once Ras-GTP levels were increased after SV40-LT expression. This observation provides support for the capability of oncolytic herpesvirus in targeting premalignant (immortalized) cells on the basis of their increased Ras activity and therefore preventing their transition to malignancy. Activation of Raf kinase has been proposed as an additional mechanism for SV40-LT immortalization.55 Because Raf is one of the most important effectors of Ras, we were interested in determining whether the inhibition of Raf kinase with a specific blocker can reduce the permissiveness of Schwann cells to herpes. We found a reduction in the amount of protein synthesis for wild-type HSV-1 and G207 was observed in cells exposed to Raf kinase inhibitor II.
Studies by Mahller and colleagues75 reviewed the permissiveness of MPNST cells to G207 in human MPNST cell lines with low-and high-Ras-GTP levels. In their study, it is reported that human MPNST cells were permissive to G207, however, the study does not provide sufficient signal transduction data on which to draw a conclusion about the relationship between Ras signaling and sensitivity to oncolytic herpesvirus. The data, however, show that the level of Ras-GTP in most of the human MPNST cells were found to be higher than their corresponding normal Schwann cells, which might explain the reason for their increased permissiveness to G207 corroborating our findings.
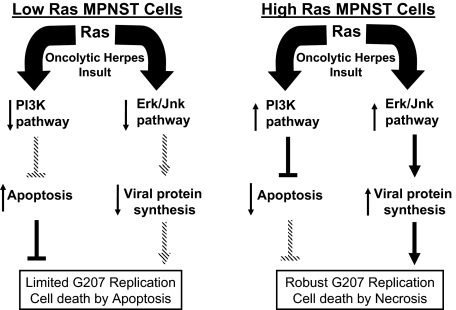
Although oncolytic viruses are usually believed to destroy cancer cells by lytic infection, our data suggest that implementation of such events is in relationship with the Ras signaling characteristics of the target cells. Figure 6 summarizes the impact of Ras signaling on the biological response of MPNST cells to G207. In case of low levels of Ras activation, the production of viral proteins does not occur at efficient levels and diminished PI3K pathway activity opens the way for occurrence of apoptosis in these cells on infection with G207. One possibility is that the existence of a signaling profile that is not conducive to viral replication can trigger other effects such as apoptosis because of the toxicity of some viral proteins. The exact mechanism involved in this phenomenon awaits clarification. In the case of high-Ras signaling MPNST cells, however, an increased level of permissiveness to virus is observed leading to lysis of cells and occurrence of necrosis. In both cases, a significant loss of viability and invasiveness is observed in our studies. In both cases, although via primarily different mechanisms, exposure to G207 induced cytotoxicity in MPNST cells. Collectively, the data obtained in our study on cells from mouse model for MPNST show that the signaling characteristics of cells, and not only their genotypic profile, is an important determinant of their behavior against an insult by oncolytic herpes.
Figure 6.
Ras signaling pathway influences the outcome of oncolytic virus G207 insult in MPNST cells. This diagram explains the influence of Ras signaling via its downstream effector pathways on permissiveness of MPNST cells to G207. In cells with low levels of Ras signaling, viral protein synthesis does not occur at an efficient level. However, exposure to virus does induce certain levels of apoptosis in these cells in part because of an inactive PI3K pathway failing to exert its anti-apoptotic effects. In cells with elevated levels of Ras signaling, however, efficient viral protein synthesis and activation of the PI3K pathway reduces the capability of cells to undergo apoptosis. The expected outcome of this scenario would be robust G207 replication and death of cells via lytic infection (necrosis).
Acknowledgments
We thank Michael Franklin for exceptional editorial assistance with this manuscript.
Footnotes
Address reprint requests to Faris Farassati, Ph.D., Pharm.D., Assistant Professor Division of Hematology, Oncology, and Transplantation, Department of Medicine, Kansas University Medical Center, 3901 Rainbow Blvd. Kansas City, Kansas, 66160. E-mail: ffarassati@kumc.edu.
Supported by the National Institutes of Health (R01-NS032677 to R.L.M.) and the Department of Defense (NF050014 to F.F.).
Conflict of Interest: S.D.R. and R.L.M. are consultants to MediGene Inc., which has a license from Georgetown University for G207. F.F. holds a material transfer agreement with MediGene Inc. for the use of G207.
References
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Cox AD, Der CJ. Ras family signaling: therapeutic targeting. Cancer Biol Ther. 2002;1:599–606. doi: 10.4161/cbt.306. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–114. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Bollag G, Freeman S, Lyons JF, Post LE. Raf pathway inhibitors in oncology. Curr Opin Investig Drugs. 2003;4:1436–1441. [PubMed] [Google Scholar]
- Jones HA, Hahn SM, Bernhard E, McKenna WG. Ras inhibitors and radiation therapy. Semin Radiat Oncol. 2001;11:328–337. doi: 10.1053/srao.2001.26020. [DOI] [PubMed] [Google Scholar]
- Eckert LB, Repasky GA, Ulku AS, McFall A, Zhou H, Sartor CI, Der CJ. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51–62. doi: 10.1023/a:1006491619920. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J. Biological and clinical significance of HER2 overexpression in breast cancer. Breast Cancer. 2001;8:45–51. doi: 10.1007/BF02967477. [DOI] [PubMed] [Google Scholar]
- Li T, Sparano JA. Inhibiting Ras signaling in the therapy of breast cancer. Clin Breast Cancer. 2003;3:405–420. doi: 10.3816/CBC.2003.n.005. [DOI] [PubMed] [Google Scholar]
- Shackney SE, Silverman JF. Molecular evolutionary patterns in breast cancer. Adv Anat Pathol. 2003;10:278–290. doi: 10.1097/00125480-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4-2 prostate cancer cells. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- Maroni PD, Koul S, Meacham RB, Koul HK. Mitogen activated protein kinase signal transduction pathways in the prostate. Cell Commun Signal. 2004;2:5. doi: 10.1186/1478-811X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Vogt A, Oguri T, Lazo JS. Activation of the Raf-1/MEK/Erk kinase pathway by a novel Cdc25 inhibitor in human prostate cancer cells. Prostate. 2004;58:95–102. doi: 10.1002/pros.10292. [DOI] [PubMed] [Google Scholar]
- Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- Moyret-Lalle C, Marcais C, Jacquemier J, Moles JP, Daver A, Soret JY, Jeanteur P, Ozturk M, Theillet C. Ras, p53 and HPV status in benign and malignant prostate tumors. Int J Cancer. 1995;64:124–129. doi: 10.1002/ijc.2910640209. [DOI] [PubMed] [Google Scholar]
- Pergolizzi RG, Kreis W, Rottach C, Susin M, Broome JD. Mutational status of codons 12 and 13 of the N- and K-ras genes in tissue and cell lines derived from primary and metastatic prostate carcinomas. Cancer Invest. 1993;11:25–32. doi: 10.3109/07357909309020257. [DOI] [PubMed] [Google Scholar]
- Firgaira FA, Seshadri R, McEvoy CR, Dite GS, Giles GG, McCredie MR, Southey MC, Venter DJ, Hopper JL. HRAS1 rare minisatellite alleles and breast cancer in Australian women under age forty years. J Natl Cancer Inst. 1999;91:2107–2111. doi: 10.1093/jnci/91.24.2107. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Holmlund J, Fleming GF, Mani S, Stadler WM, Schumm P, Monia BP, Johnston JF, Geary R, Yu RZ, Kwoh TJ, Dorr FA, Ratain MJ. Phase I trial of ISIS 5132, an antisense oligonucleotide inhibitor of c-raf-1, administered by 24-hour weekly infusion to patients with advanced cancer. Clin Cancer Res. 2001;7:1214–1220. [PubMed] [Google Scholar]
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin. 2002;20:841–865. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- Frahm S, Kurtz A, Kluwe L, Farassati F, Friedrich RE, Mautner VF. Sulindac derivatives inhibit cell growth and induce apoptosis in primary cells from malignant peripheral nerve sheath tumors of NF1-patients. Cancer Cell Int. 2004;4:4. doi: 10.1186/1475-2867-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet. 1999;89:23–30. doi: 10.1002/(sici)1096-8628(19990326)89:1<23::aid-ajmg6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Lloyd AC. Ras/Raf/ERK signalling and NF1. Cell Cycle. 2004;3:1255–1258. doi: 10.4161/cc.3.10.1182. [DOI] [PubMed] [Google Scholar]
- Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- Bajenaru ML, Donahoe J, Corral T, Reilly KM, Brophy S, Pellicer A, Gutmann DH. Neurofibromatosis 1 (NF1) heterozygosity results in a cell-autonomous growth advantage for astrocytes. Glia. 2001;33:314–323. doi: 10.1002/1098-1136(20010315)33:4<314::aid-glia1030>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275:30740–30745. doi: 10.1074/jbc.M001702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra E, Puig S, Otero D, Gaona A, Kruyer H, Ars E, Estivill X, Lazaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997;61:512–519. doi: 10.1086/515504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci USA. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legius E, Dierick H, Wu R, Hall BK, Marynen P, Cassiman JJ, Glover TW. TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosom Cancer. 1994;10:250–255. doi: 10.1002/gcc.2870100405. [DOI] [PubMed] [Google Scholar]
- Rey JA, Pestana A, Bello MJ. Cytogenetics and molecular genetics of nervous system tumors. Oncol Res. 1992;4:321–331. [PubMed] [Google Scholar]
- Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant. G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Moulding HD, Chahlavi A, Khan GA, Rabkin SD, Martuza RL, Driever PH, Kurtz A. Therapeutic efficacy of G207 in a novel peripheral nerve sheath tumor model. Exp Neurol. 2001;169:64–71. doi: 10.1006/exnr.2001.7641. [DOI] [PubMed] [Google Scholar]
- Farassati F, Lee PW. Ras signalling pathway: a gateway for HSV-1 infection. Sci World J. 2003;3:533–535. doi: 10.1100/tsw.2003.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3:745–750. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Norman KL, Coffey MC, Hirasawa K, Demetrick DJ, Nishikawa SG, DiFrancesco LM, Strong JE, Lee PW. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Downward J. Ras activation of phosphatidylinositol 3-kinase and Akt. Methods Enzymol. 2001;333:37–44. doi: 10.1016/s0076-6879(01)33042-2. [DOI] [PubMed] [Google Scholar]
- Al-Mohanna MA, Al-Khodairy FM, Krezolek Z, Bertilsson PA, Al-Houssein KA, Aboussekhra A. p53 is dispensable for UV-induced cell cycle arrest at late G(1) in mammalian cells. Carcinogenesis. 2001;22:573–578. doi: 10.1093/carcin/22.4.573. [DOI] [PubMed] [Google Scholar]
- Kumlin T, Heikkinen P, Kosma VM, Alhonen L, Janne J, Juutilainen J. p53-independent apoptosis in UV-irradiated mouse skin: possible inhibition by 50 Hz magnetic fields. Radiat Environ Biophys. 2002;41:155–158. doi: 10.1007/s00411-002-0153-8. [DOI] [PubMed] [Google Scholar]
- Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim JA, Chai KJ. Molecular assembly of mitogen-activated protein kinase module in ras-transformed NIH3T3 cell line. Exp Mol Med. 2000;32:120–126. doi: 10.1038/emm.2000.21. [DOI] [PubMed] [Google Scholar]
- Jha PK, Beral V, Peto J, Hack S, Hermon C, Deacon J, Mant D, Chilvers C, Vessey MP, Pike MC. Antibodies to human papillomavirus and to other genital infectious agents and invasive cervical cancer risk. Lancet. 1993;341:1116–1118. doi: 10.1016/0140-6736(93)93128-n. [DOI] [PubMed] [Google Scholar]
- Raptis L, Brownell HL, Corbley MJ, Wood KW, Wang D, Haliotis T. Cellular ras gene activity is required for full neoplastic transformation by the large tumor antigen of SV40. Cell Growth Differ. 1997;8:891–901. [PubMed] [Google Scholar]
- Grammatikakis N, Jaronczyk K, Siganou A, Vultur A, Brownell HL, Benzaquen M, Rausch C, Lapointe R, Gjoerup O, Roberts TM, Raptis L. Simian virus 40 large tumor antigen modulates the Raf signaling pathway. J Biol Chem. 2001;276:27840–27845. doi: 10.1074/jbc.M101987200. [DOI] [PubMed] [Google Scholar]
- Brunet A, Pouyssegur J. Mammalian MAP kinase modules: how to transduce specific signals. Essays Biochem. 1997;32:1–16. [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Martín-Blanco E. p38 MAPK signalling cascades: ancient roles and new functions. Bioessays. 2000;22:637–645. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- Plattner R, Gupta S, Khosravi-Far R, Sato KY, Perucho M, Der CJ, Stanbridge EJ. Differential contribution of the ERK and JNK mitogen-activated protein kinase cascades to Ras transformation of HT1080 fibrosarcoma and DLD-1 colon carcinoma cells. Oncogene. 1999;18:1807–1817. doi: 10.1038/sj.onc.1202482. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Marte BM, Warne PH, Downward J. Phosphatidylinositol 3′ kinase: one of the effectors of Ras. Philos Trans R Soc Lond B Biol Sci. 1996;351:222–231. doi: 10.1098/rstb.1996.0020. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Feldkamp MM, Angelov L, Guha A. Neurofibromatosis type 1 peripheral nerve tumors: aberrant activation of the Ras pathway. Surg Neurol. 1999;51:211–218. doi: 10.1016/s0090-3019(97)00356-x. [DOI] [PubMed] [Google Scholar]
- McCormick F. Ras signaling and NF1. Curr Opin Genet Dev. 1995;5:51–55. doi: 10.1016/s0959-437x(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Norman KL, Farassati F, Lee PW. Oncolytic viruses and cancer therapy. Cytokine Growth Factor Rev. 2001;12:271–282. doi: 10.1016/s1359-6101(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Wildner O. Oncolytic viruses as therapeutic agents. Ann Med. 2001;33:291–304. doi: 10.3109/07853890109002081. [DOI] [PubMed] [Google Scholar]
- Andreansky S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- Jorgensen TJ, Katz S, Wittmack EK, Varghese S, Todo T, Rabkin SD, Martuza RL. Ionizing radiation does not alter the antitumor activity of herpes simplex virus vector g207 in subcutaneous tumor models of human and murine prostate cancer. Neoplasia. 2001;3:451–456. doi: 10.1038/sj.neo.7900193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome JT, Platenberg RC, Manz HJ, Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S, Newsome JT, Rabkin SD, McGeagh K, Mahoney D, Nielsen P, Todo T, Martuza RL. Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum Gene Ther. 2001;12:999–1010. doi: 10.1089/104303401750195944. [DOI] [PubMed] [Google Scholar]
- Shmulevitz M, Marcato P, Lee PW. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- Kalbacova M, Spisakova M, Liskova J, Melkova Z. Lytic infection with vaccinia virus activates caspases in a Bcl-2-inhibitable manner. Virus Res. 2008;135:53–63. doi: 10.1016/j.virusres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Mahller YY, Vaikunth SS, Currier MA, Miller SJ, Ripberger MC, Hsu YH, Mehrian-Shai R, Collins MH, Crombleholme TM, Ratner N, Cripe TP. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15:279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]