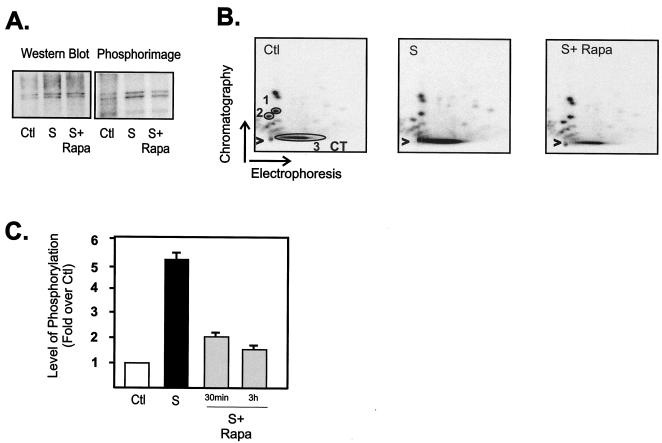
FIG. 5.
mTOR regulates phosphorylation of the UBF carboxy-terminal activation domain. NIH 3T3 cells were made quiescent (serum starved as a control), stimulated with serum (grown in DMEM containing 10% FBS for 24 h), and then treated with rapamycin (20 nM) or vehicle (ethanol) for 30 min or 3 h. The cells were also labeled with 1 mCi of 32Pi per plate 12 h after serum was added. 32P-labeled UBF was immunoprecipitated, separated by SDS-PAGE, and transferred to Immobilon P membranes. The membranes were then subjected to Western blot analysis using anti-UBF antibodies and phosphorimager analysis (A). Alternatively, 32P-labeled UBF was excised and digested with trypsin, and the resultant peptides were separated by electrophoresis and chromatography as described in Materials and Methods. Radiolabeled phosphopeptides were visualized after 24-h exposure using a PhosphorImager (B). Panels A and B show typical results for a 3-h rapamycin treatment. (C) The results from two or three separate experiments incorporating 30-min and 3-h treatment with rapamycin were quantitated using ImageQuant software (Molecular Dynamics), and the level of carboxy-terminal domain (CT) phosphorylation was expressed relative to the phosphorylation of peptides 1 and 2 (circled 1 and 2 in panel B). Abbreviations: Ctl, control; S, 10% FBS; S+ Rapa, 10% FBS plus 20 nM rapamycin.