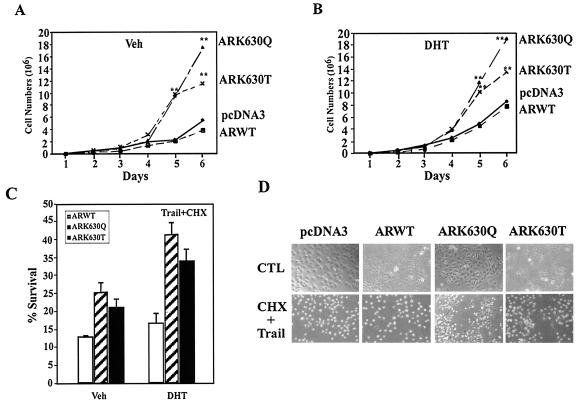
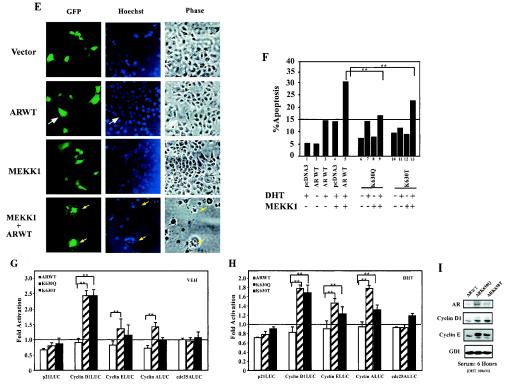
FIG. 6.
AR acetylation site governs cellular proliferation and MEKK1-dependent apoptosis. (A and B) Enhanced cellular proliferation rate of AR acetylation site mutants. A total of 2 × 104 DU145 cells stably transfected with the expression vector for either wild-type AR, AR mutants, or control vector pcDNA3 were seeded and treated either with vehicle (A) or DHT (10−7M) (B). The representative results of three independent experiments are shown. (C) AR acetylation site prostate cancer cell lines are resistant to TRAIL-induced apoptosis. Cell survival rates of DU145 stable cell lines exposed to TRAIL (10 ng/ml) and cycloheximide (CHX; 10 μg/ml) are shown compared with untreated cells (100%). (D) Phase contrast of cell lines is shown. (E, F). AR acetylation site mutants evade MEKK-1-mediated apoptosis. DU145 cells were transfected with MEKK1, AR, and pCMV-GFP as indicated and treated with either vehicle or DHT (10 nM) for 24 h. The morphology of the transfected DU145 cells is shown in phase contrast. (E) White arrows indicate GFP-positive cells, and yellow arrows indicate GFP-positive cells showing chromatin condensation. (F) The graph represents independent experiments in which 200 green fluorescent cells were counted and scored for cytoplasmic blebbing and chromatin condensation; *, P < 0.01 for the effect of MEKK1 on liganded AR-induced apoptosis. (G and H) AR acetylation site mutants alter regulation of cell cycle control genes. Reporter assays showing regulation of cell cycle control promoters by wild-type AR or AR acetylation site mutants in DU145 cells treated with vehicle (G) or DHT (H). (I) Western blot analysis of DU145 stable cell lines stably expressing wild-type AR and AR acetylation mutants. Cells were starved for 24 h and harvested 6 h after treatment (10% charcoal-stripped fetal bovine serum plus 100 nM DHT). GDI served as a protein-loading control.