Abstract
Development of hematopoietic cells in the aorta-gonad-mesonephros (AGM) region in the midgestation mouse embryo involves a multistep process, sequentially changing from endothelial cell-like cells, including hemangioblasts, into hematopoietic stem cells, progenitors, and/or lineage-committed cells. An adaptor molecule, Lnk, is known to negatively control the production of pro- and pre-B cells and hematopoietic progenitor cells in adult bone marrow. Here we show a role of Lnk in hematopoietic development in the AGM region. Lnk was predominantly expressed in the endothelial cells lining the dorsal aorta at embryonic day 11.5 (E11.5). Overexpression of Lnk in the primary culture of the AGM region at E11.5 suppressed the emergence of CD45+ hematopoietic cells. Point mutation in the SH2 domain of Lnk, which abolishes the binding capability of Lnk to c-Kit upon stimulation with stem cell factor (SCF), led to loss of Lnk-dependent inhibition of hematopoietic cell development in AGM cultures, suggesting Lnk-mediated inhibition of the SCF/c-Kit signaling pathway. In cultured AGM cells from Lnk homozygous mutant mouse embryos, the number of emerged CD45+ cells was 2.5-fold larger than that from heterozygous littermates. Furthermore, aorta cells of E11.5 Lnk homozygous mutant mice also showed enhanced hematopoietic colony-forming activity. Thus, Lnk is a negative regulator of hematopoiesis in the AGM region.
Hematopoietic stem cells are the source of all mature blood cells, erythrocytes, granulocytes, monocytes, platelets, and lymphocytes (13, 31). In early development in the mouse, hematopoiesis first arises from the blood islands of the yolk sac at embryonic day 7.5 (E7.5) and subsequently occurs in the para-aortic splanchnopleura region at E7.5 to 9.5, in parallel with the yolk sac, and in the aorta-gonad-mesonephros (AGM) region at E10.5 to 11.5 (4, 15, 16, 23). Hematopoietic stem cells are thought to migrate to the fetal liver and further emigrate to the spleen and bone marrow by the time of birth (6). Long-term repopulating hematopoietic stem cells, which express several marker proteins, emerge in the AGM region of the mouse at midgestation. In vitro differentiation of embryonic stem (ES) cells gives rise to a population of definitive hematopoietic cells via endothelial precursor cells (3, 7, 25, 27). These observations indicated the possibility that hematopoietic stem cells in early definitive hematopoiesis differentiate from the endothelial precursor cells and/or hemangioblasts, which are the precursors of both hematopoietic and endothelial cells.
Endothelial cells are known to differentiate into hematopoietic cells in the primary culture system of the AGM region at E10.5 to 11.5 (22). When the AGM region of the mouse embryo at E11.5 was cultured with cytokines such as stem cell factor (SCF), basic fibroblast growth factor (bFGF), and oncostatin M (OSM), the endothelial cell-like cells are first evident after a few days of culture, and then nonadherent cells including hematopoietic progenitors are detected and gradually increase in the culture (22). In the culture of the AGM region from mouse embryos lacking the transcription factor c-Myb and Runx1 (AML1, Cbfa2, and Pebp2αB), which are known to be important for hematopoiesis, endothelial cell-like cells are generated but not hematopoietic cells (20, 21). These results are in accord with the in vivo phenotype of mice lacking c-Myb or Runx1 and in vitro developmental experiments using Runx1-deficient ES cells (11). Moreover, introduction of cDNA for c-Myb or Runx1 into the cultured AGM from these mutant embryos partially restored the production of nonadherent cells (20, 21).
Lnk is composed of a number of functional regions, including the N-terminal region, which is likely to be required for multimerization, the pleckstrin homology domain, which is suggested to have a role in binding to phospholipids or other proteins, the Src homology 2 (SH2) domain, which is known to be critical for specific binding to a phosphotyrosine residue, and a Tyr phosphorylation motif that is phosphorylated in response to SCF. Lnk is thus suggested to have a role as an adaptor protein. It was originally reported that tyrosine-phosphorylated Lnk is bound to the SH2 domain of Grb2, phospholipase C γ-1, and phosphatidylinositol 3-kinase in activated T cells (8) and functions as a negative mediator of the T-cell receptor signaling pathway (12). However, development and activation of T cells are normal in Lnk-deficient mice. In contrast, these mice exhibit a significant increase in the number of pre-B cells in the spleen and pre- and pro-B cells in the bone marrow, indicating that Lnk has an important role in regulating B-cell development (40). In a more recent study on Lnk-deficient mice, Takaki et al. demonstrated that the mice display a significant increase in hematopoietic progenitor cells in the adult bone marrow (39).
Lnk has structural similarities to APS and SH2-B, which both contain the multimerization, pleckstrin homology, and SH2 domains, and these three proteins form a family of adaptor proteins (32, 40, 45). APS and SH2-B have been reported to associate with insulin receptor (1, 10, 18, 34), Trk family receptors (33, 36), platelet-derived growth factor receptor (35, 46), and tyrosine kinase Janus kinase (JAK) (37, 44). APS is tyrosine phosphorylated in response to B-cell antigen receptor stimulation (9). The existence of four SH2-B splice variants (α, β, γ, and δ) derived from the same gene has recently been reported (47).
In the present study, we observed the expression of Lnk in the AGM region of the E11.5 mouse embryo, in particular in the endothelium of the dorsal aorta. We also show that introduction of Lnk into the primary culture of the AGM region resulted in inhibition of the generation of hematopoietic cells. This inhibition was abolished by a defect in the binding of the SH2 domain of Lnk to the c-Kit cytoplasmic region. We further demonstrated that the number of the hematopoietic cells is increased in the AGM culture derived from homozygous Lnk mutant mice compared to heterozygotes. Our results reveal that the Lnk adaptor protein negatively regulates AGM hematopoiesis.
MATERIALS AND METHODS
Tissue culture.
The AGM regions were excised at E11.5 from ICR or Lnk-targeted embryos and trypsinized. Cells were suspended in Dulbecco's modified Eagle's medium supplemented with 15% (vol/vol) fetal calf serum and cultured in gelatin-coated 12- or 24 well-plates in the presence of 100 ng of murine SCF (Pepro Tech Inc, Rocky Hill, N.J.), 1 ng of bFGF (R&D Systems, Minneapolis, Minn.), and 10 ng of OSM (R&D Systems) per ml.
Retrovirus infection of cells.
cDNA was inserted into a pMY-IRES-EGFP vector. Plat-E cells for packaging ecotropic retrovirus were plated the night before transfection (19). Transient transfection of Plat-E cells with plasmid DNA was performed with Trans IT-293 reagent (Mirus, Madison, Wis.) according to the manufacturer's protocol. Cells were incubated for 48 h and the supernatant was collected and used for infection. The viral supernatant was added together with 1 μg of Polybrene per ml and cytokines as described above. After incubation for 9 h, the virus-containing medium was replaced with standard growth medium. Infected cells were confirmed by the fluorescence of green fluorescent protein (GFP).
Semisolid colony-forming assays.
Nonadherent cells in the AGM culture and the trypsinized primary aorta cells of E11.5 embryos were suspended in minimal essential medium, alpha modification, containing 0.8% (wt/vol) methylcellulose, 30% fetal calf serum, 1% deionized bovine serum albumin, 100 μM 2-mercaptoethanol, 20 ng of murine interleukin-3 (IL-3)/ml, 100 ng of SCF/ml, and 4 U of erythropoietin (EPO)/ml. Cells were cultured in triplicate in 35-mm dishes at 37°C for 7 days. Individual colonies, GFP+ cells, and all cells were scored by morphology.
Flow cytometry.
After being washed in phosphate-buffered saline (PBS) containing 3% (vol/vol) fatal calf serum and 0.05% sodium azide, the nonadherent cells in the AGM culture were incubated for 30 min on ice with phycoerythrin-conjugated rat anti-mouse CD45 (30-F11) (Becton Dickinson, Lincoln, N.J.) and analyzed by FACSCalibur (Becton Dickinson). The percentage of the CD45+ cells in the GFP+ cells was determined.
Nonadherent cells were reacted with biotinylated anti-mouse Mac1 (M1/70), Gr1 (RB6-8C5), Ter119 (TER119), B220 (RA3-6B2), CD4 (RM4-5), and CD8 (53-6.7) (Becton Dickinson). After washing, cells were incubated with streptavidin-conjugated magnetic beads (Miltenyi Biotech). Lin− cells (Mac-1− Gr-1− Ter119− B220− CD4− CD8−) were separated with the MACS system (Mirus). Isolated cells were incubated with phycoerythrin-conjugated anti-mouse Sca-1 (E13-161.7) and allophycocyanin-conjugated anti-mouse c-Kit (2B8) (Becton Dickinson) and analyzed. The percentage of c-Kit+ and Sca-1+ cells in the GFP+ cells was determined.
RT-PCR.
Total RNAs were isolated from E9.5, 11.5, and 14.5 aorta, E14.5 fetal liver, and adult mouse muscle of adult mouse (negative control). cDNAs were synthesized with 5 μg of total RNAs as templates in 20 μl of the reaction mixture with Superscript II reverse transcriptase (Gibco-BRL, Rockville, Md.). PCRs were carried out with rTaq (Takara) with the following settings: 95°C for 3 min and 26 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 1 min. The primer sets used were as follows: 5′-CTCAAGGAGGTCGTATTGCGCTA-3′, 5′-TTCCAGTGGGATAGGAGAACGC-3′ (for Lnk); 5′-GAGACGACGACAGCGGTGGGTGCT-3′, 5′-GATGGGGTGGGTGTGGAAGTGACG-3′ (for APS); 5′-GAGGGGCCTCCAGCAGGGACA-3′, 5′-GCCTCTTCTGCCCCAGGATGT-3′ (for SH2-B); 5′-ACCACAGTCCATGCCATCAC-3′, 5′-TCCACCACCCTGTTGCTGTA-3′ (for glyceraldehyde-3-phosphate dehydrogenase).
Immunohistochemical staining.
Mouse embryos at E11.5 were fixed in 2% (wt/vol) paraformaldehyde-PBS overnight at 4°C, equilibrated in 20% sucrose-PBS for 2 h at 4°C, quick-frozen in Tissue Tek, and stored at −70°C. Sections (5 μm) were washed with PBS and blocked in 3% fetal calf serum-PBS. Sections were then stained with control rabbit IgG, anti-Lnk (the C-terminal region of Lnk) (41), or anti-CD34 (RAM34) (Becton Dickinson) for 2 h at room temperature, followed by washing and treatment with a rhodamine-conjugated secondary antibody for 2 h. After washing, bisbenzimide H33258 fluorochrome trihydrochloride (Nacalai Tesque, Kyoto, Japan) was used to stain the nuclei.
Immunoblotting and coimmunoprecipitation analysis.
Cultured AGM cells (6 × 105) were infected with mock or Flag-tagged Lnk mutant retroviruses and a c-Kit retrovirus. On the next day, cells were starved for 16 h and then treated with SCF (100 ng/ml) for 20 min. After 20 min, cells were dissolved with the lysis buffer (0.5% Nonidet-40, 10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM EDTA, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin/ml). Lysates were immunoprecipitated with anti-Flag antibody (M2; Sigma, St. Louis, Mo.) or anti-c-Kit antibody (M-14; Santa Cruz Biotechnology, Santa Cruz, Calif.). The immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-c-Kit antibody or anti-Flag antibody. Labeled proteins were detected with an enhanced chemiluminescence system (ECL, Amersham Bioscience Corp, Piscataway, N.J.).
Luciferase assay.
Elk activation was measured by the GAL-4 DNA-binding domain (DB)/Elk-1 fusion system according to the manufacturer's protocol (PathDetect in vitro signal transduction pathway trans-reporting system, Stratagene). Briefly, 293 cells (0.8 × 105) plated on 12-well plates were transfected with Elk-1 consisting of GAL-4 DB and Elk-1 (25 ng), pFR-Luc carrying the GAL-4 upstream activation sequence-fused luciferase gene (50 ng), pRL-CMV encoding the sea pansy luciferase gene (25 ng), c-Kit (67.5 ng, for SCF stimulation), and Lnk expression vectors (0.1, 0.3, 1, 3, or 10 ng) with Trans-IT 293 (Mirus). On the following day, cells were stimulated with SCF (20 ng/ml), bFGF (1 ng/ml), or IL-6/soluble IL-6 receptor (sIL-6R) (20 ng/ml) for 6 h and then solubilized. Luciferase activities in cell lysates were assessed with the Pikkagene dual luciferase assay system (Tokyo Ink Inc., Chuou-ku, Tokyo, Japan) and a MicroLumat LB96P luminometer (Berthold Technologies GmbH & Co. KG, Calmbacher, Bad Wild, Germany).
RESULTS
Expression and localization of Lnk in the AGM region.
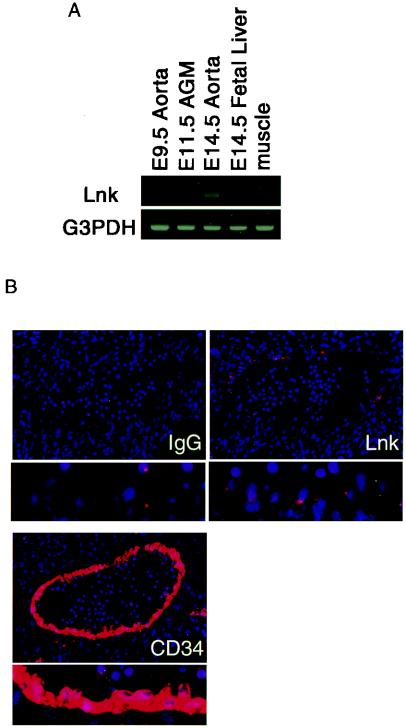
We first examined the expression of Lnk in fetal hematopoietic sites by RT-PCR (Fig. 1A). Transcripts for Lnk were detectable in the AGM region at E9.5, 11.5, and 14.5 and the fetal liver at E14.5, but not in the muscle of adult mice. We further analyzed the expression of Lnk protein in the AGM region at E11.5 by immunohistochemical staining of transverse sections (Fig. 1B). Lnk was present in the endothelial cells lining the dorsal aorta. These results indicate that Lnk is expressed in the AGM region at a stage of embryonic hematopoiesis. The expression pattern of Lnk overlapped with that of CD34 in the dorsal aorta, suggesting that Lnk might be involved in hematopoietic cell development from endothelial precursors.
FIG. 1.
Expression of Lnk in the AGM region. (A) Total RNAs were extracted from E9.5, 11.5, and 14.5 aorta, E14.5 fetal liver, and adult mouse muscle and then subjected to RT-PCR with specific primers for Lnk. (B) Tissue sections of E11.5 embryos were stained with control IgG antibody, anti-Lnk polyclonal antibody, and anti-CD34 monoclonal antibody. The lower panel shows a higher magnification view of endothelial cells. Endothelial cells lining the dorsal aorta are stained with anti-Lnk antibody. G3PDH, glyceraldehyde-3-phosphate dehydrogenase.
Inhibition of hematopoietic differentiation in the AGM cultures by Lnk.
Recent studies have shown that primary culture of the AGM region from mouse embryos at E11.5 can be used to examine the mechanism by which hematopoiesis of multipotential progenitors occurs in vitro (20-22, 28, 29). Addition of SCF, bFGF, and OSM to this culture system is required for the efficient expansion of hematopoietic progenitors from endothelial cell-like cells. To examine whether Lnk functions in the production of hematopoietic cells from the endothelial cell-like cells, we retrovirally overexpressed Lnk protein in the AGM culture. Lnk cDNA was ligated into a retroviral vector containing an internal ribosomal entry sequence (IRES) motif linked to the enhanced GFP (EGFP) to the track the transduced cells. Adherent cells in a 2-day AGM culture were infected with this retrovirus, and the infection was monitored by GFP expression.
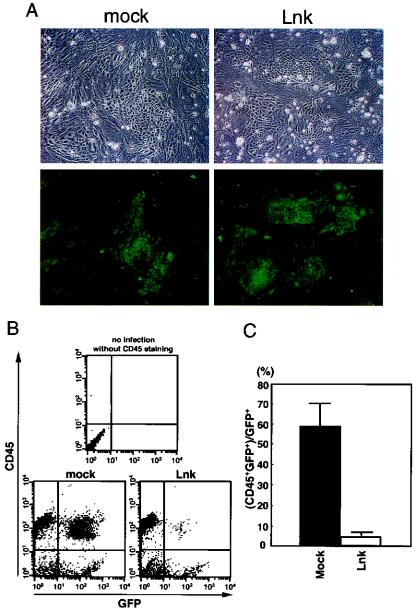
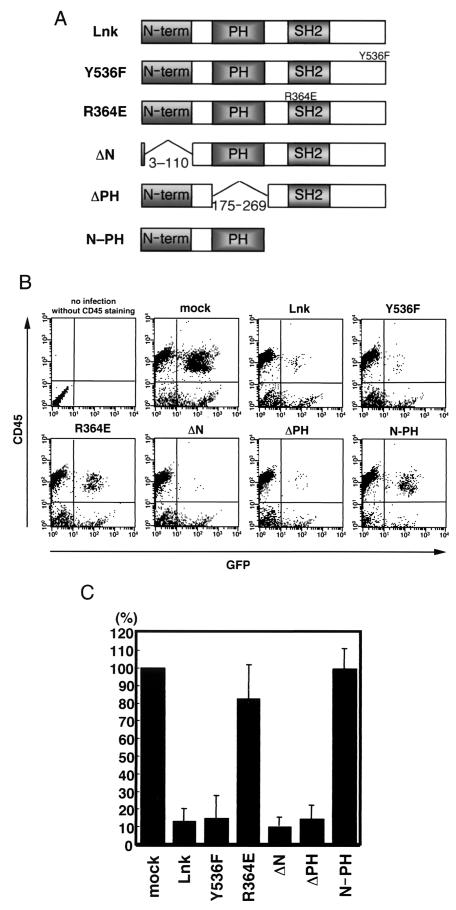
Five days after infection, Lnk- and vehicle-infected adherent cells showed similar morphology and viability, as examined after removal of nonadherent cells by moderate pipetting (Fig. 2A). The expression of Lnk protein from the Lnk-IRES-GFP retrovirus in the infected cells was confirmed by immunoblotting (see Fig. 5). Expression of GFP in vehicle- and Lnk-infected cases was observed in the adherent cells, especially in the endothelial cell-like cells (Fig. 2A). These results indicate that retrovirally expressed Lnk has no influence on the growth or survival of the adherent cells in the AGM culture. Five days after infection, nonadherent cells in the AGM cultures were analyzed by flow cytometry with monoclonal antibodies against CD45, which is a marker of hematopoietic cells except for erythrocytes. As shown in Fig. 2B, overexpression of Lnk resulted in a dramatic reduction in CD45-positive cells. The percentage of CD45+ GFP+ cells to total GFP+ cells in the Lnk-infected culture was only 4.3%, which was in marked contrast to that in the vehicle-infected culture (57.8%) (Fig. 2C). This result implies an inhibitory function of Lnk for hematopoietic differentiation in the AGM cultures.
FIG. 2.
Inhibition of hematopoietic differentiation by Lnk in cultured AGM cells. (A) E11.5 AGM cells were cultured with SCF (100 ng/ml), bFGF (1 ng/ml), and OSM (10 ng/ml). On day 2 of culture, cells were infected with GFP and/or Lnk retrovirus. On day 7 of culture, adherent cells in the vehicle- and Lnk-infected AGM cultures showed normal proliferation, and no significant difference in the number of the GFP+ adherent cells between these cultures was observed. (B) On day 7 of culture, nonadherent cells were stained with anti-CD45 antibody, and 104 cells were analyzed by flow cytometry. Representative plots of four independent experiments are shown. (C) The percentage of the CD45+ cells in the GFP+ cells was determined. Error bars indicate the standard error of the mean (n = 4).
FIG. 5.
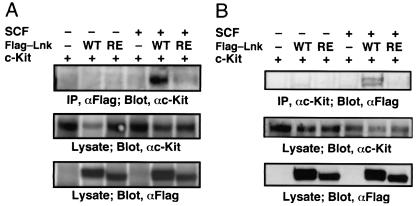
Requirement of the SH2 domain of Lnk for interaction with c-Kit in cultured AGM cells. (A and B) Cultured AGM cells were infected with retroviruses encoding either Flag-tagged Lnk or Lnk-R364E together with a retrovirus encoding c-Kit. The cells were stimulated with SCF (100 ng/ml) for 20 min. Cell extracts were subjected to immunoprecipitation with anti-Flag antibody (A) and anti-c-Kit antibody (B). Precipitates or lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-c-Kit antibody (A) and anti-Flag antibody (B). The expression of Lnk and c-Kit was monitored.
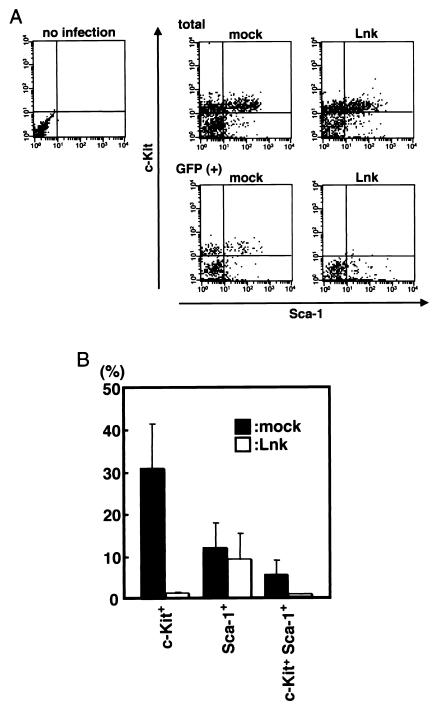
Next, to further examine the effects of Lnk on AGM hematopoiesis, lineage marker-negative cells were isolated from the nonadherent cells in AGM cultures by negative selection and stained with c-Kit and Sca-1 antibodies. It has been reported that Lin− c-Kit+ Sca-1+ cells contain most of the long-term multilineage reconstituting activities in the murine bone marrow. As shown in Fig. 3, the Lin− c-Kit+ Sca-1+ GFP+ cell fraction was markedly decreased in the Lnk-infected AGM cultures. The percentages of nonadherent cells that expressed c-Kit in the GFP-positive cells were 1.3% of Lnk-infected cells and 31.7% of vehicle-infected cells (Fig. 3B), suggesting that Lnk inhibits the appearance of c-Kit+ cells.
FIG. 3.
Lnk-driven inhibition of the appearance of c-Kit on cultured AGM cells. (A) E11.5 AGM cells were cultured with SCF, bFGF, and OSM. On day 2 of culture, cells were infected with GFP or Lnk retrovirus. On day 7 of culture, Lin− cells were separated from nonadherent cells with magnetic microbeads. Purified cells were stained with anti-c-Kit and Sca-1 antibodies and analyzed by flow cytometry. In each flow cytometric profile, 3 × 103 (total cells, upper three panels) or 5 × 102 (GFP+ cells, lower two panels) events are recorded. (B) The percentage of c-Kit+ and Sca-1+ cells in the 5 × 102 GFP+ cells was determined. Error bars indicate the standard error of the mean (n = 5).
Furthermore, the colony-forming activity of nonadherent cells from the AGM culture was assayed in methylcellulose medium containing SCF, IL-3, and EPO. The Lnk-expressing nonadherent cells derived from the AGM cultures were unable to form colonies containing granulocytes, macrophages, and erythrocytes (Table 1). These results indicate that Lnk has a role in the negative regulation of hematopoiesis in the AGM, at least in culture.
TABLE 1.
Colony-forming activities in AGM-derived nonadherent cells in culturea
| Cell source for colony assay | Infection | No. of colonies per 105 nonadherent cells
|
||||
|---|---|---|---|---|---|---|
| CFU-G | CFU-M | CFU-GM | CFU-E | CFU-Mix | ||
| GFP+ cells | Mock | 9.8 ± 5.3 | 58.0 ± 2.3 | 1.8 ± 1.0 | 0.0 ± 0.0 | 0.7 ± 0.3 |
| Lnk | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| GFP+/− cells | Mock | 85.9 ± 7.2 | 605.3 ± 8.9 | 16.8 ± 2.3 | 11.3 ± 4.5 | 8.5 ± 3.3 |
| Lnk | 109.0 ± 7.2 | 699.3 ± 10.0 | 20.3 ± 1.8 | 5.5 ± 6.1 | 5.5 ± 2.8 | |
Nonadherent cells expanded in the retrovirus-infected AGM cultures were inoculated in methylcellulose medium containing SCF (100 ng/ml), IL-3 (20 ng/ml), and EPO (4U/ml). Colonies were scored at 7 days of culture. Colony types: G, granulocyte; M, macrophage; GM, granulocyte and macrophage; E, erythrocyte; Mix, graneulocyte, macrophage, erythrocyte, and megakaryocyte. Results represent the mean ± standard error of triplicate samples.
Essential role for the SH2 domain of Lnk in hematopoietic differentiation in AGM culture.
To determine the region important for the inhibitory activity of Lnk, we constructed a series of point and deletion mutants of Lnk: a mutant (Y536F) with a substitution of Phe for Tyr536 which is phosphorylated in response to SCF stimulation (39); a mutant (R346E) with a substitution of Glu for the conserved Arg in the SH2 domain which is known to abolish binding to a phosphotyrosine-containing target; and mutants devoid of the N-terminal region (ΔN), the pleckstrin homology domain (ΔPH), and the C-terminal half (N-PH). As shown in Fig. 4, R364E and N-PH did not work as inhibitors of hematopoiesis. These data suggest that the SH2 domain of Lnk is essential for inhibition of hematopoiesis in the AGM culture.
FIG. 4.
Ability of various Lnk mutants to inhibit hematopoietic differentiation in cultured AGM cells. (A) The structures of the Lnk mutants are represented schematically. (B) E11.5 AGM cells were cultured with SCF (100 ng/ml), bFGF (1 ng/ml), and OSM (10 ng/ml). On day 2 of culture, cells were infected with retrovirus encoding GFP and/or Lnk mutants. On day 7 of culture, nonadherent cells were stained with anti-CD45 antibody, and 104 cells were analyzed by flow cytometry. (C) The percentages of CD45+ cells among the Lnk mutant-expressing GFP+ cells were determined. These data were normalized to the value in the mock culture which was set at 100%. Error bars indicate the standard error of the mean (n = 4).
It has been shown that Lnk is associated with the SCF receptor c-Kit (39). To examine the molecular mechanism of interaction between Lnk and c-Kit, we expressed wild-type Lnk, R364E mutant, and c-Kit proteins in the AGM culture. SCF stimulation induced the association between Lnk and c-Kit in the AGM culture, and the R364E substitution resulted in a loss of their interaction (Fig. 5). Thus, the cytoplasmic region of c-Kit likely conforms to the binding site of the SH2 domain of Lnk in the intermolecular interaction, which raises the possibility that hematopoiesis in the AGM culture was blocked by the inhibition of the c-Kit signal caused by the interaction of Lnk.
Effect of Lnk on SCF, bFGF, and OSM signaling.
We have previously shown that a stable transfectant of Lnk attenuated activation of mitogen-activated protein kinase as detected by phosphorylation of Erk upon stimulation with SCF (39). To evaluate the functional role of Lnk in SCF-, bFGF-, and OSM-induced signaling, we examined the effect of Lnk on the activation of Elk-1, one of the nuclear targets of Erk, reporter activity with 293 cells. 293 cells transfected with an Elk-1 reporter were used for this experiment. Since 293 cells do not express OSM receptors but express gp130, the signal transducing receptor component of the OSM receptor complex, IL-6/sIL-6R fusion protein was used in place of OSM because IL-6/sIL-6R is known to stimulate gp130. It should be noted that IL-6/sIL-6R and OSM have similar biological effects on the AGM culture (42).
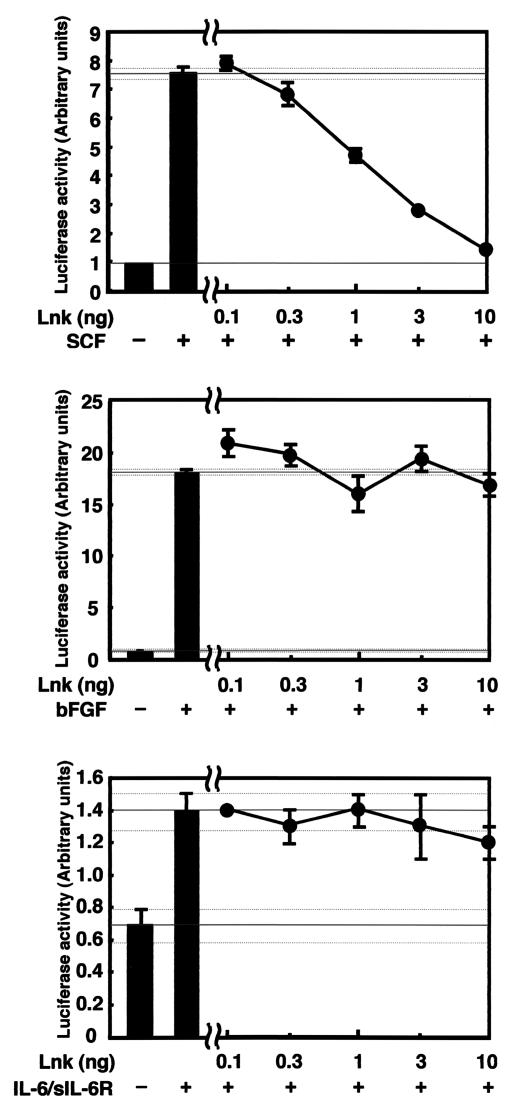
First, the 50% effective concentrations of the cytokines SCF, bFGF, and IL-6/sIL-6R for Elk-1 reporter activity were determined by dose-response studies: SCF, 20 ng/ml; bFGF, 1 ng/ml; and IL-6/sIL-6R, 20 ng/ml (data not shown). The effect of Lnk was examined in the AGM culture with either SCF, bFGF, or IL-6/sIL-6R at concentrations of 20, 1, and 20 ng/ml, respectively. As shown in Fig. 6, Lnk dose-dependently inhibited SCF-induced Elk-1 activation. In contrast, Lnk did not significantly affect bFGF- and IL-6/sIL-6R-induced Elk-1 activation (Fig. 6). These results suggest that Lnk selectively inhibited the SCF-induced Erk pathway activation.
FIG. 6.
Effect of Lnk on SCF-, bFGF-, and IL-6/sIL-6R-induced Elk-1 reporter activity. 293 cells were transfected with an Elk-1 reporter plasmid and the indicated amounts of Lnk plasmid. In the experiment with SCF, the c-Kit plasmid was also used. The cells were stimulated on the following day with either SCF (20 ng/ml), bFGF (1 ng/ml), or IL-6/sIL-6R (20 ng/ml) for 6 h, and luciferase activities in the cell lysates were measured.
Ability of Lnk family proteins, APS and SH2-B isoforms, to inhibit hematopoietic differentiation in cultured AGM cells.
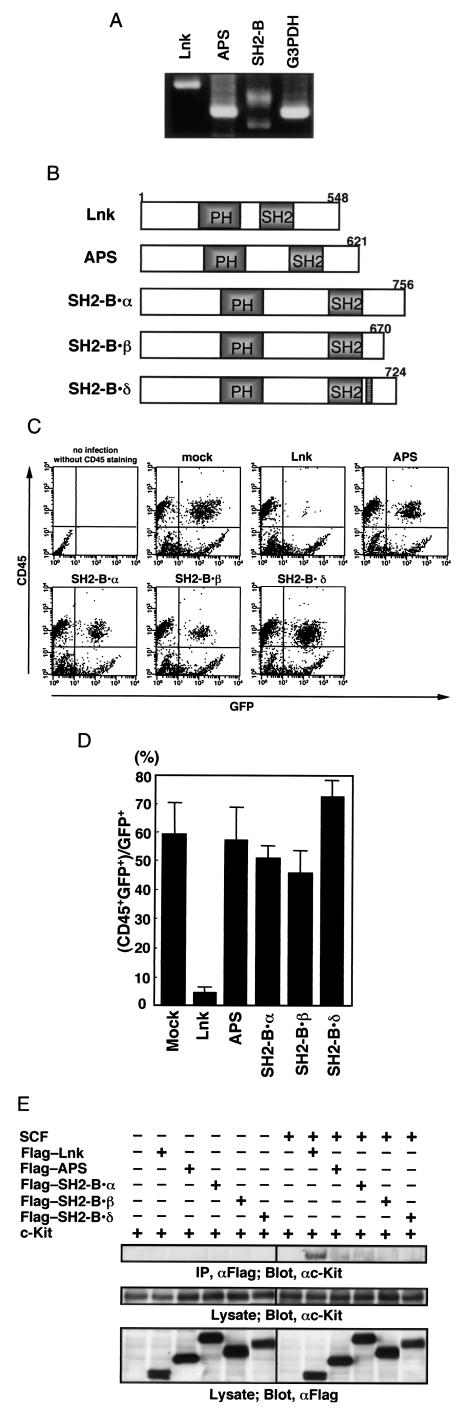
We examined the effects of the Lnk family proteins APS and SH2-B splicing variants on hematopoietic differentiation in cultured AGM cells. We first analyzed Lnk family protein expression in the AGM region at E11.5 by RT-PCR. As shown in Fig. 7A, transcripts for APS and a common part of the SH2-B splicing variants were observed in the AGM region at E11.5. Next, to investigate functional differences in the inhibitory activities of Lnk family proteins, we expressed Lnk, APS, and SH2-B isoforms (α, β, and δ) with retroviruses in AGM cultures (Fig. 7B). Overexpression of either APS or SH2-B isoforms could not inhibit the proliferation of the CD45+ cells, indicating that the inhibitory effect of Lnk on the AGM-cultured cells was not generally applicable to other Lnk family proteins (Fig. 7C and D). Furthermore, we examined SCF-mediated interaction between Lnk family proteins and c-Kit in the AGM culture. Lnk bound strongly to c-Kit in the presence of SCF, but no other family members showed significant binding (Fig. 7E). Based on these observations, it seems that Lnk is highly effective in regulating hematopoiesis in AGM cells.
FIG.7.
Ability of Lnk family proteins to inhibit hematopoietic differentiation in cultured AGM cells. (A) Expression of Lnk family proteins in the AGM region. Total RNAs were extracted from E11.5 aorta and subjected to RT-PCR with specific primers for each indicated molecule. (B) The structures of Lnk, APS, and SH2-B splicing variants (SH2-B α, β, and δ) are represented schematically. (C) E11.5 AGM cells were cultured with cytokines as described above. On day 2 of culture, cells were infected with retroviruses encoding GFP or Lnk family proteins. On day 7 of culture, nonadherent cells were stained with anti-CD45 antibody, and 104 cells were analyzed by flow cytometry. (D) The percentage of CD45+ cells in the GFP+ cells was determined. Error bars indicate the standard error of the mean (n = 3). (E) Flag-tagged Lnk family proteins were transfected into cultured AGM cells with a retrovirus carrying c-Kit. Cells were stimulated with SCF (100 ng/ml) for 20 min. Cell extracts were subjected to immunoprecipitation with anti-Flag antibody. Precipitates or lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-c-Kit antibody. The expression of Lnk and c-Kit was monitored.
Role of Lnk in AGM hematopoiesis.
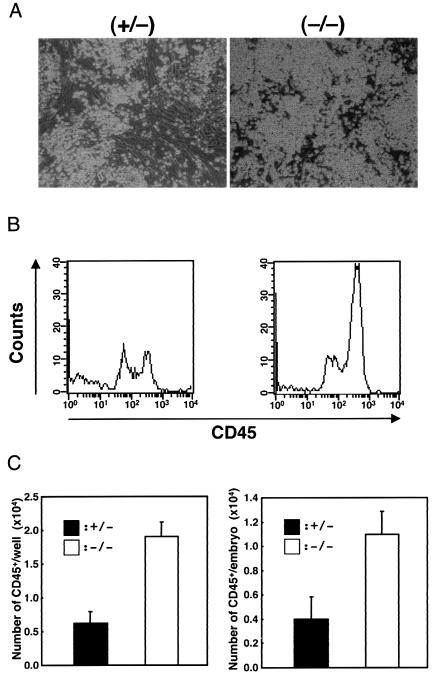
In light of these results obtained from the Lnk overexpression experiments, we examined the effect of Lnk deficiency on embryonic hematopoiesis with AGM cells from E11.5 Lnk +/− and Lnk −/− littermates. As shown in Fig. 8A, a larger number of nonadherent cells were observed in Lnk −/− AGM cultures than in Lnk+/− cultures, while the expansion of adherent cells in Lnk −/− cultures was comparable to that in Lnk+/− cultures. Moreover, flow cytometric analysis showed that the expression of CD45 increased in nonadherent cells from Lnk −/− AGM cultures (Fig. 8B). The numbers of CD45+ nonadherent cells in each well and that in each embryo were both greater in Lnk −/− than in Lnk+/− mice (Fig. 8C).
FIG. 8.
Induction of hematopoietic differentiation in the Lnk−/− AGM culture. (A) Dynamic expansion of nonadherent cells from the Lnk−/− AGM culture. E11.5 AGM cells from Lnk+/− and Lnk−/− littermates were plated on gelatin-coated 24-well plates at a density of 1.5 × 105 per well with cytokines as described above. The proliferation rates of the adherent cells in the Lnk−/− and Lnk+/− cultures were comparable. After 7 days of culture, nonadherent cells were generated and counted. (B) Flow cytometric profiles of the nonadherent cells (104 cells) from the AGM culture stained with anti-CD45 antibody. (C) The numbers of CD45+ nonadherent cells in each well (left) or each embryo (right) were determined by the percentages of CD45+ cells in the nonadherent cells. Error bars indicate the standard error of the mean (n = 3).
Furthermore, the emergence of colony-forming activities of nonadherent cells was examined in the in vitro AGM culture. The frequency of nonadherent cells forming a colony in Lnk −/− mice was approximately twofold higher than that in Lnk+/− mice (Table 2). These results indicate that the AGM region of E11.5 Lnk−/− mice had a higher capacity to differentiate into hematopoietic cells than did that of Lnk+/− mice. To examine the ability of AGM cells with Lnk deficiency to form hematopoietic colonies, we performed the same experiments with the exception of omitting the 7-day culture. In contrast to the primary AGM cells of heterozygous littermates (Lnk+/−), those of homozygous mice (Lnk−/−) showed increased capability for colony formation (Table 3). The data suggest a negative regulatory role of Lnk in AGM hematopoiesis in vivo.
TABLE 2.
Colony-forming activities in AGM-derived nonadherent cell culture in Lnk-deficient micea
| Cell source for colony assay | No. of colonies per 2 × 105 nonadherent cells
|
||||
|---|---|---|---|---|---|
| CFU-G | CFU-M | CFU-GM | CFU-E | CFU-Mix | |
| Lnk+/− cells | 9.0 ± 2.6 | 51.3 ± 1.2 | 1.0 ± 0.0 | 0.7 ± 0.6 | 0.3 ± 0.6 |
| Lnk−/− cells | 21.3 ± 5.7 | 105.3 ± 8.1 | 1.7 ± 0.6 | 1.0 ± 1.0 | 0.3 ± 0.6 |
Nonadherent cells expanded in the AGM cultures of Lnk-deficient mice were inoculated in methylcellulose medium containing SCF (100 ng/ml), IL-3 (20 ng/ml), and EPO (4 U/ml). Colonies were scored at 7 days of culture. Colony types: G, granulocyte; M, macrophage; GM, granulocyte and macrophage; E, erythrocyte; Mix, graneulocyte, macrophage, erythrocyte, and megakaryocyte. Results represent the mean ± standard error of triplicate samples.
TABLE 3.
Colony-forming activities in primary E11.5 aorta cells in Lnk-deficient micea
| Cell source for colony assay | No. of colonies per 2 × 105 primary E11.5 aorta cells
|
||||
|---|---|---|---|---|---|
| CFU-G | CFU-M | CFU-GM | CFU-E | CFU-Mix | |
| Lnk+/− | 22.5 ± 4.7 | 107.3 ± 17.6 | 3.5 ± 2.1 | 2.3 ± 1.0 | 14.8 ± 3.0 |
| Lnk−/− | 58.5 ± 13.8 | 175.5 ± 25.1 | 6.3 ± 3.0 | 2.5 ± 1.0 | 20.8 ± 3.6 |
Primary E11.5 aorta cells from Lnk+/− and Lnk−/− littermates were inoculated in methylcellulose medium containing SCF (100 ng/ml), IL-3 (20 ng/ml), and EPO (4 U/ml). Colonies were scored at 7 days of culture. Colony types: G, granulocyte; M, macrophage; GM, granulocyte and macrophage; E, erythrocyte; Mix, graneulocyte, macrophage, erythrocyte, and megakaryocyte. Results represent the mean ± standard error of quadruplicate samples.
DISCUSSION
In the present study, Lnk was shown to function as a negative regulator of the development of hematopoiesis in the AGM region during mouse embryogenesis. We demonstrated that Lnk had inhibitory effects on hematopoietic differentiation in the AGM culture and colony-forming ability and that these inhibitory effects of Lnk were mediated by the binding of the Lnk SH2 domain to the phosphorylated c-Kit receptor.
Cultured AGM cells derived from E11.5 mouse embryos retain many of the characteristics of in vivo hematopoiesis at this stage. In this culture system, endothelial cell-like cells, including hemangioblasts, are thought to expand and subsequently generate nonadherent hematopoietic progenitors (Lin− c-Kit+ Sca-1+) and then CD45+ hematopoietic cells. Finally, these nonadherent cells display the morphology and markers of specific lineages. From our results, the introduction of a retrovirus encoding Lnk-IRES-GFP into endothelial cell-like cells decreased the number of cells expressing c-Kit and CD45. The proliferation of GFP+ adherent cells in the AGM culture was unaffected by introduction of Lnk compared to that in the vehicle-infected cells (Fig. 2A), and expression of Flag-tagged Lnk in the AGM culture was confirmed by immunoblotting analysis (Fig. 5).
Recently, Ly-6A, a component of Sca-1 protein in mice, was shown to be expressed in the single cell layer lining the dorsal aorta at E11.5 (5), while c-Kit expression is found in the ventral wall of the dorsal aorta and hematopoietic cell clusters, which attach to the ventral wall of the dorsal aorta. The expression pattern of Runx1/AML-1 in the endothelial cells of the dorsal aorta at E11.5 shows similarity with that of c-Kit, while the clusters of CD45+ cells are also present on the ventral side (26). Considering the expression patterns of these molecules, endothelial cells in the midgestation mouse aorta are suggested to have long-term reconstitution activities. c-Kit+ cells are observed in all hematopoietic stem cells in the AGM region (38). We demonstrated that Lnk was expressed in the endothelial cells lining the dorsal aorta (Fig. 1B), and the introduction of Lnk significantly reduced the number of c-Kit-positive cells (Fig. 3), suggesting that Lnk inhibited the differentiation of endothelial cells (hemangioblasts) into hematopoietic progenitors and lineage-committed cells.
In the previous studies, AGM cultures from transcriptional factor c-Myb or Runx1 null embryos, which die at E15.5 and E12.5, respectively, have not produced hematopoietic cells (21, 22). In op/op mice with defective macrophage colony-stimulating factor, the expansion of hematopoietic progenitors and the reduction in the expression of endothelial markers were observed in the AGM culture (17). Mice deficient for Lnk are born normally and show no developmental abnormalities in appearance (38) but had an enhanced colony-forming ability of the primary AGM cells at E11.5, as well as that for nonadherent cells from cultured AGM cells (Tables 2 and 3) and exhibited an increase in the number of hematopoietic progenitors (Lin− c-Kit+ Sca-1+) in the adult bone marrow (39).
Our data raise a couple of possibilities for the precise physiological function of Lnk in AGM hematopoiesis: a modulation of the timing of hematopoietic differentiation from the AGM regions by controlling SCF/c-Kit signaling and negative regulation of SCF/c-Kit signaling at the time of the movement of a site of definitive hematopoiesis from the AGM region to the fetal liver. Further studies regarding the molecular mechanisms of transcriptional regulation and these functions of Lnk should better clarify hematopoiesis during embryonic development.
We consider that Lnk acts through the c-Kit receptor to regulate the growth and differentiation of hematopoietic cells. Mukoyama et al. have shown that OSM is required for the development of hematopoietic progenitors in the AGM culture in the presence of SCF and bFGF (22). When cultured in serum-free medium with cytokine, E11.5 AGM cells were able to expand and differentiate into hematopoietic progenitor cells (data not shown). In serum-free culture, the nonadherent cells were sufficiently generated by the addition of only SCF, compared with those given SCF, bFGF, and OSM. This result indicates that the SCF/c-Kit signaling pathway is essential for the growth and differentiation of hematopoiesis in the AGM culture. Moreover, Lnk selectively inhibited the SCF signaling pathway, at least in 293 cells (Fig. 6). It is most likely that the overexpression of Lnk eventually led to the inhibition of the SCF/c-Kit signaling pathway in the AGM culture.
Lnk is an adaptor protein containing some functional regions or domains: the N-terminal putative multimerization region, the pleckstrin homology domain involved in interaction with phospholipids or other proteins, the SH2 domain capable of binding to phosphotyrosine residues, and the tyrosine 536 residue subjected to phosphorylation upon SCF stimulation (32, 39). As shown in the overexpression experiment with a series of Lnk mutants in the AGM culture, the SH2 domain of Lnk was a prerequisite for inhibition of the generation of CD45+ nonadherent cells (Fig. 4). In addition, the SH2 domain of Lnk was involved in the interaction with c-Kit receptors (Fig. 5), suggesting that SCF-induced signaling might be inhibited by Lnk via the SH2 domain. SCF-induced tyrosine phosphorylation of c-Kit receptors generates the binding site for signal-transducing proteins, for example, Grb2, the p85 subunits of phosphatidylinositol 3-kinase, phospholipase C-γ1, and Src kinase, and consequently leads to proliferation, survival, calcium mobilization, cell migration, and differentiation (2, 14, 43). The mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways are known to be initiated by SCF stimulation. Expansion of nonadherent cells from AGM cultures was dose-dependently inhibited by the mitogen-activated protein kinase/ERK kinase inhibitor U0126 (I. Nobuhisa and T. Taga, unpublished data). A recent study has shown that a stable transformant of Lnk attenuates phosphorylation of Gab2 and activation of the mitogen-activated protein kinase pathway with an SCF-dependent mast cell line, MC9 (39). In present study, Lnk suppressed SCF-induced Erk activation (Fig. 6). Moreover, the SCF-induced phosphorylation levels of ERK and Akt (downstream of phosphatidylinositol 3-kinase) are reduced in Gab2−/− mast cells (24). These observations raise the possibility that Lnk blocks mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways by associating with c-Kit receptors.
Lnk is a member of the Lnk protein family including, for example, APS and SH2-B isoforms. Functional specificity is observed in Lnk family protein-deficient mice. Lnk-deficient mice exhibit a significant increase in the number of hematopoietic progenitors in the adult bone marrow (39, 40). In SH2-B-deficient mice, the numbers of follicles and sperms are both reduced compared with the wild type, resulting in small genital organs, ovaries, and testes (30). Among the Lnk family proteins, Lnk and APS have previously been reported to bind to c-Kit upon stimulation with SCF (9, 39, 46). Here we demonstrated that Lnk had inhibitory effects on AGM hematopoiesis but that APS and SH2-B did not, and, moreover, that a strong interaction of the SH2 domain of Lnk with c-Kit was observed in AGM culture. These results indicate that Lnk, but not APS or SH2-B, regulates AGM hematopoiesis.
c-Myb and Runx1 are known to play a positive regulatory role in the emergence of hematopoietic cells in the AGM culture (20, 21), whereas Lnk is revealed to function as a negative regulator in the present study. Since retrovirally expressed Lnk inhibited the appearance of GFP+ CD45+ cells in the AGM culture, the expression and/or transcriptional activity of c-Myb and Runx1 might be affected by Lnk. Negative regulators of cytokine signaling are most likely to modulate the amount or functional properties of transcription factors that are essential for AGM hematopoiesis.
Acknowledgments
We thank A. Yoshimura for providing SH2-B cDNA, M. Iseki for APS cDNA, Y. Kanakura for c-Kit cDNAs, T. Kitamura for pMY-IRES-GFP and Plat-E cells, and T. Kagawa, T. Kondo, and K. Nakashima for valuable discussions. We also thank Y. Noguchi for secretarial assistance and K. Kaneko for technical help.
This work was supported in part by a Grant-in-Aid for 21st Century COE Research from the Ministry of Education, Culture, Sports, Science and Technology “Cell Fate Regulation Research and Education Unit,” the Human Frontier Science Program, and the Virtual Research Institute of Aging of Nippon Boehringer Ingelheim.
REFERENCES
- 1.Ahmed, Z., B. J. Smith, K. Kotani, P. Wilden, and T. S. Pillay. 1999. APS, an adapter protein with a pleckstrin homology and SH2 domain, is a substrate for the insulin receptor kinase. Biochem. J. 341:665-668. [PMC free article] [PubMed] [Google Scholar]
- 2.Broudy, V. C. 1997. Stem cell factor and hematopoiesis. Blood 90:1345-1364. [PubMed] [Google Scholar]
- 3.Chung, Y. S., W. J. Zhang, E. Arentson, P. D. Kingsley, J. Palis, and K. Choi. 2002. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development 129:5511-5520. [DOI] [PubMed] [Google Scholar]
- 4.Cumano, A., F. Dieterlen-Lievre, and I. Godin. 1996. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86:907-916. [DOI] [PubMed] [Google Scholar]
- 5.de Bruijin, M. R. T. R., X. Ma, C. Robin, K. Ottersbatch, M. J. Sanchez, and E. Dzierzak. 2002. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16:673-683. [DOI] [PubMed] [Google Scholar]
- 6.Ema, H., and H. Nakauchi. 2000. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95:2284-2288. [PubMed] [Google Scholar]
- 7.Hirashima, M., H. Kataoka, S. Nishikawa, and N. Matsuyoshi. 1999. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood 93:1253-1263. [PubMed] [Google Scholar]
- 8.Huang, X., Y. Li, K. Tanaka, K. G. Moore, and J. I. Hayashi. 1995. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase Proc. Natl. Acad. Sci. 92:11618-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iseki, M., S. Takaki, and K. Takatsu. 2000. Molecular cloning of the mouse APS as a member of the Lnk family adaptor proteins. Biochem. Biophys. Res. Commun. 272:45-54. [DOI] [PubMed] [Google Scholar]
- 10.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J. 335:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacaud, G., L. Gore, M. Kennedy, V. Kouskoff, P. Kingsley, C. Hogan, L. Carlsson, N. Speck, J. Palis, and G. Keller. 2002. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 100:458-466. [DOI] [PubMed] [Google Scholar]
- 12.Li, Y., X. He, J. Schembri-King, S. Jakes, and J. Hayashi. 2000. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T-cell activation. J. Immunol. 164:5199-5206. [DOI] [PubMed] [Google Scholar]
- 13.Ling, K. W., and E. Dzierzak. 2002. Ontogeny and genetics of the hemato/lymphopoietic system. Curr. Opin. Immunol. 14:186-191. [DOI] [PubMed] [Google Scholar]
- 14.Linnekin, D. 1999. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 31:1053-1074. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka, S., K. Tsuji, H. Hisakawa, M. Xu, Y. Ebihara, T. Ishii, D. Sugiyama, A. Manabe, R. Tanaka, Y. Ikeda, S. Asano, and T. Nakahata. 2001. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood 98:6-12. [DOI] [PubMed] [Google Scholar]
- 16.Medvinsky, A., and E. Dzierzak. 1996. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86:897-906. [DOI] [PubMed] [Google Scholar]
- 17.Minehata, K., Y. S. Mukouyama, T. Sekiguchi, T. Hara, and A. Miyajima. 2002. Macrophage colony stimulating factor modulates the development of hematopoiesis by stimulating the differentiation of endothelial cells in the AGM region. Blood 99:2360-2368. [DOI] [PubMed] [Google Scholar]
- 18.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 19.Morita, S., T. Kojima, and T. Kitamura. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7:1063-1066. [DOI] [PubMed] [Google Scholar]
- 20.Mukouyama, Y., N. Chiba, T. Hara, H. Okada, Y. Ito, R. Kanamaru, A. Miyajima, M. Satake, and T. Watanabe. 2000. The AML1 transcription factor functions to develop and maintain hematogenic precursor cells in the embryonic aorta-gonad-mesonephros region. Dev. Biol. 220:27-36. [DOI] [PubMed] [Google Scholar]
- 21.Mukouyama, Y., N. Chiba, M. L. Mucenski, M. Satake, A. Miyajima, T. Hara, and T. Watanabe. 1999. Hematopoietic cells in cultures of the murine embryonic aorta-gonad-mesonephros region are induced by c-Myb. Curr. Biol. 9:833-836. [DOI] [PubMed] [Google Scholar]
- 22.Mukouyama, Y., T. Hara, M. Xu, K. Tamura, P. J. Donovan, H. Kim, H. Kogo, K. Tsuji, T. Nakahata, and A. Miyajima. 1998. In vitro expansion of murine multipotential hematopoietic progenitors from the embryonic aorta-gonad-mesonephros region. Immunity 8:105-114. [DOI] [PubMed] [Google Scholar]
- 23.Muller, A. M., A. Medvinsky, J. Strouboulis, F. Grosveld, and E. Dzierzak. 1994. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1:291-301. [DOI] [PubMed] [Google Scholar]
- 24.Nishida, K., L. Wang, E. Morii, S. J. Park, M. Narimatsu, S. Itoh, S. Yamasaki, M. Fujishima, K. Ishihara, M. Hibi, Y. Kitamura, and T. Hirano. 2002. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99:1866-1869. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa, S. I., S. Nishikawa, M. Hirashima, N. Matsuyoshi, and H. Kodama. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747-1757. [DOI] [PubMed] [Google Scholar]
- 26.North, T. E., M. R. T. R. de Bruijin, T. Stacy, L. Talebian, E. Lind, C. Robin, M. Binder, E. Dzierzak, and N. A. Speck. 2002. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16:661-672. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa, M., M. Kizumoto, S. Nishikawa, T. Fujimoto, H. Kodama, and S. I. Nishikawa. 1999. Expression of alpha4-integrin defines the earliest precursor of hematopoietic cell lineage diverged from endothelial cells. Blood 93:1168-1177. [PubMed] [Google Scholar]
- 28.Ohmura, K., H. Kawamoto, S. Fujimoto, S. Ozaki, K. Nakao, and Y. Katsura. 1999. Emergence of T, B, and myeloid lineage-committed as well as multipotent hemopoietic progenitors in the aorta-gonad-mesonephros region of day 10 fetuses of the mouse. J. Immunol. 163:4788-4795. [PubMed] [Google Scholar]
- 29.Ohmura, K., H. Kawamoto, M. Lu, T. Ikawa, S. Ozaki, K. Nakao, and Y. Katsura. 2001. Immature multipotent hemopoietic progenitors lacking long-term bone marrow-reconstituting activity in the aorta-gonad-mesonephros region of murine day 10 fetuses. J. Immunol. 166:3290-3296. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsuka, S., S. Takaki, M. Iseki, K. Miyoshi, N. Nakagata, Y. Kataoka, N. Yoshida, K. Takatsu, and A. Yoshimura. 2002. SH2-B is required for both male and female reproduction Mol. Cell. Biol. 22:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orkin, S. H., and L. I. Zon. 2002. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nat. Immunol. 3:323-328. [DOI] [PubMed] [Google Scholar]
- 32.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 34.Riedel, H., J. Wang, H. Hansen, and N. Yousaf. 1997. PSM, an insulin-dependent, pro-rich, pleckstrin homology, SH2 domain containing partner of the insulin receptor. J. Biochem. (Tokyo) 122:1105-1113. [DOI] [PubMed] [Google Scholar]
- 35.Rui, L., and C. Carter-Su. 1998. Platelet-derived growth factor (platelet-derived growth factor) stimulates the association of SH2-Bbeta with platelet-derived growth factor receptor and phosphorylation of SH2-Bbeta. J. Biol. Chem. 273:21239-21245. [DOI] [PubMed] [Google Scholar]
- 36.Rui, L., J. Herrington, and C. Carter-Su. 1999. SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 274:26485-26492. [DOI] [PubMed] [Google Scholar]
- 37.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez, M. J., A. Holmes, C. Miles, and E. Dzierzak. 1996. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity 5:513-525. [DOI] [PubMed] [Google Scholar]
- 39.Takaki, S., H. Morita, Y. Tezuka, and K. Takatsu. 2002. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein Lnk. J. Exp. Med. 195:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaki, S., K. Sauer, B. M. Iritani, S. Chien, Y. Ebihara, K. Tsuji, K. Takatsu, and R. M. Perlmutter. 2000. Control of B cell production by the adaptor protein Lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaki, S., J. D. Watts, K. A. Forbush, N. T. Nguyen, J. Hayashi, J. Alberola-Ila, R. Aebersold, and R. M. Perlmutter. 1997. Characterization of Lnk. An adaptor protein expressed in lymphocytes. J. Biol. Chem. 272:14562-14570. [DOI] [PubMed] [Google Scholar]
- 42.Takizawa, M., I. Nobuhisa, K. Igarashi, M. Ueno, K. Nakashima, T. Kitamura, and T. Taga. 2003. Requirement of gp130 signaling for the AGM hematopoiesis. Exp. Hematol. 31:283-289. [DOI] [PubMed] [Google Scholar]
- 43.Ueda, S., M. Mizuki, H. Ikeda, T. Tsujimura, I. Matsumura, K. Nakano, H. Daino, Z. Honda Zi, J. Sonoyama, H. Shibayama, H. Sugahara, T. Machii, and Y. Kanakura. 2002. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood 99:3342-3349. [DOI] [PubMed] [Google Scholar]
- 44.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing Pleckstrin homology (pleckstrin homology) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 45.Yokouchi, M., R. Suzuki, M. Masuhara, S. Komiya, A. Inoue, and A. Yoshimura. 1997. Cloning and characterization of APS, an adaptor molecule containing pleckstrin homology and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene 15:7-15. [DOI] [PubMed] [Google Scholar]
- 46.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Yasukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing pleckstrin homology and SH2 domains, is associated with the platelet-derived growth factor receptor and c-Cbl and inhibits platelet-derived growth factor-induced mitogenesis. Oncogene 18:759-767. [DOI] [PubMed] [Google Scholar]
- 47.Yousaf, N., Y. Deng, Y. Kang, and H. Riedel. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 276:40940-40948. [DOI] [PubMed] [Google Scholar]