FIG. 4.
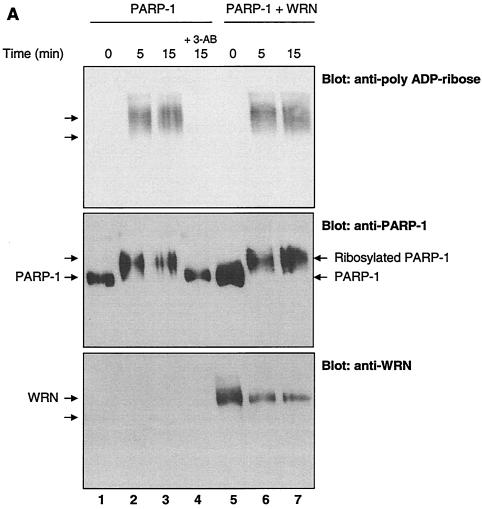
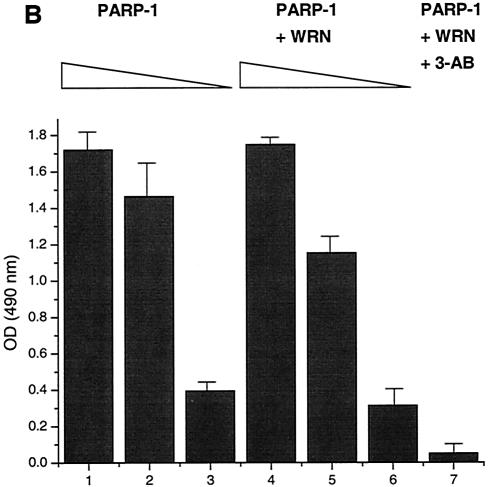
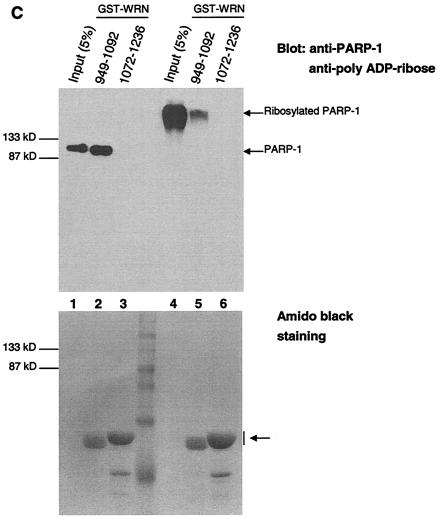
The autocatalytic activity of PARP-1 is unaffected by WRN in vitro. (A) Poly(ADP-ribosyl)ation activity of recombinant PARP-1 either alone (lanes 1 to 3) or with the inhibitor 3-AB (lane 4) or recombinant WRN (lanes 5 to 7). The reactions were terminated at the indicated times, and the samples were analyzed by SDS-10% PAGE and Western blotting with anti-PAR antibodies (top), anti-PARP-1 antibodies (middle), and anti-WRN antibodies (bottom). Arrows, SDS-PAGE mobilities of unmodified WRN and PARP-1. The increased amount of WRN detected in lane 5 is not due to the lack of reactivity of the anti-WRN antibodies but rather reflects larger amounts of WRN and PARP-1 loaded (compare lane 5 with 6 and 7 [middle and bottom]). (B) H1 histones were used to coat ELISA wells as described in Materials and Methods. After the blocking step, either 500 (bars 1, 4, and 7), 50 (bars 2 and 5), or 5 nM (bars 3 and 6) recombinant PARP-1 was added in the absence (bars 1 to 3) or presence (bars 4 to 7) of WRN (80 nM). The poly(ADP-ribosyl)ation reaction was initiated by adding NAD+ and activated DNA for 30 min at RT. The specific PARP inhibitor 3-AB was added to the ribosylation reaction (bar 7) as a negative control. The PAR polymer was detected with anti-PAR antibodies and horseradish peroxidase-conjugated secondary antibodies. The bound antibodies were detected by colorimetric analysis. OD, optical density. (C) Poly(ADP-ribosyl)ation of PARP-1 strongly decreases, but does not abolish, binding to the WRN RQC domain. GST-WRN RQC (aa 949 to 1092) (lanes 2 and 5) and GST-WRN (aa 1072 to 1236) (lanes 3 and 6) were incubated for 2 h at RT with either recombinant PARP-1 (lanes 1 to 3) or auto-poly(ADP-ribosyl)ated PARP-1 (lanes 4 to 6). The bound proteins were eluted and analyzed by SDS-7.5% PAGE and Western blotting with a mixture of anti-PARP-1 and anti-PAR antibodies. Amido black staining (bottom) shows the amount of GST-WRN fusion proteins (arrow) loaded.