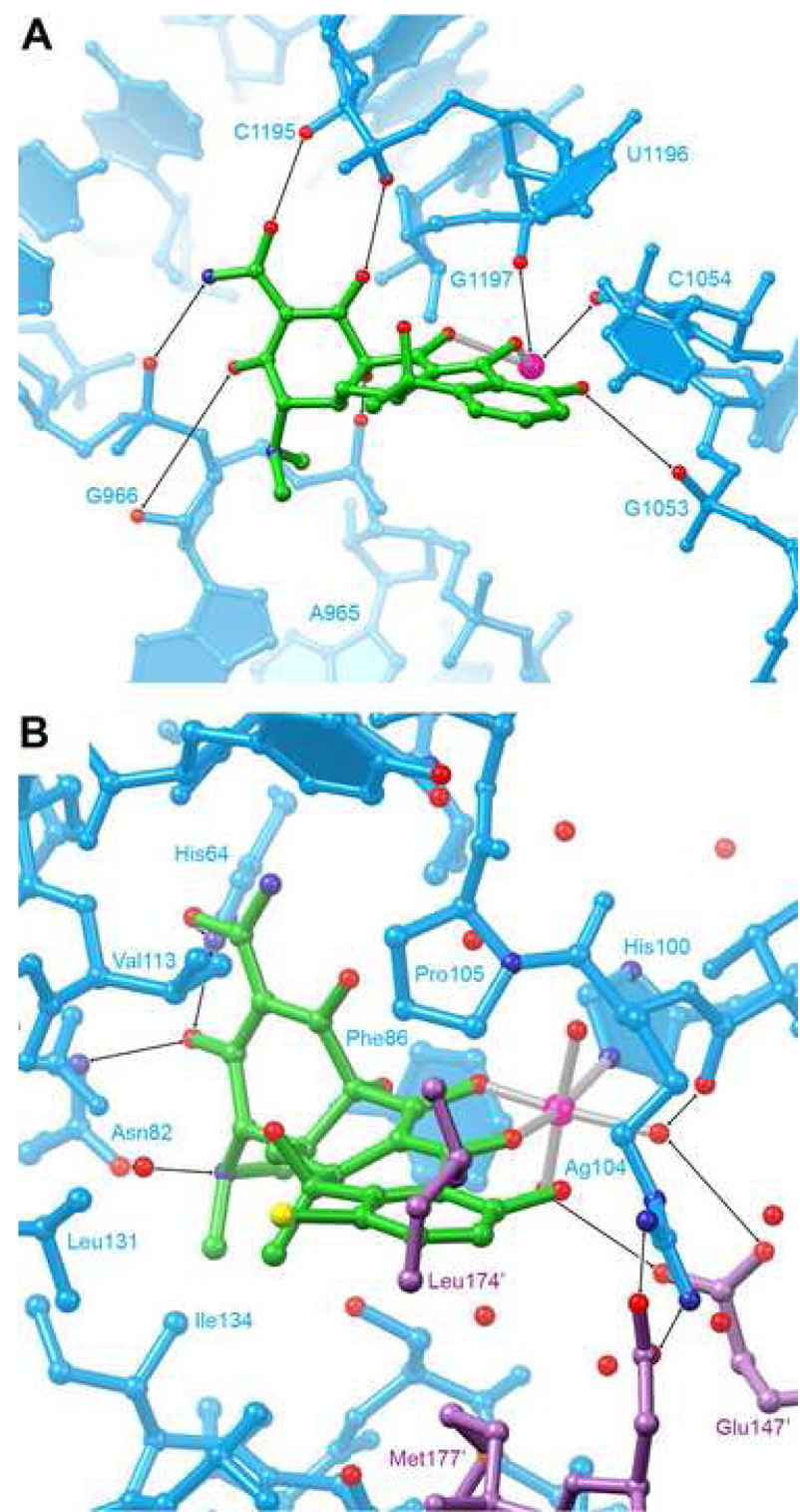
Figure 6. Comparison of ribosomal and protein tetracycline binding sites.
A, Detail of the primary tetracycline binding site in the 3.4 Å-resolution cocrystal structure of the 30S ribosomal subunit of Thermus thermophilus (Brodersen et al., 2000). B, Detail of the ligand binding site of the 2.1 Å-resolution structure of the complex between the class D tetracycline repressor protein (TetR) and 7-chlorotetracycline (Hinrichs et al., 1994; Kisker et al., 1995). The binding site is formed by amino acid residues from both protomers of the dimeric protein. Amino acid residues from the second protomer are colored magenta.