Abstract
The in vivo requirements for human natural killer (NK) cell development and differentiation into cytotoxic effectors expressing inhibitory receptors for self–major histocompatability complex class I (MHC-I; killer Ig-like receptors [KIRs]) remain undefined. Here, we dissect the role of interleukin (IL)-15 in human NK cell development using Rag2−/−γc−/− mice transplanted with human hematopoietic stem cells. Human NK cell reconstitution was intrinsically low in this model because of the poor reactivity to mouse IL-15. Although exogenous human IL-15 (hIL-15) alone made little improvement, IL-15 coupled to IL-15 receptor α (IL-15Rα) significantly augmented human NK cells. IL-15–IL-15Rα complexes induced extensive NK cell proliferation and differentiation, resulting in accumulation of CD16+KIR+ NK cells, which was not uniquely dependent on enhanced survival or preferential responsiveness of this subset to IL-15. Human NK cell differentiation in vivo required hIL-15 and progressed in a linear fashion from CD56hiCD16−KIR− to CD56loCD16+KIR−, and finally to CD56loCD16+KIR+. These data provide the first evidence that IL-15 trans-presentation regulates human NK cell homeostasis. Use of hIL-15 receptor agonists generates a robust humanized immune system model to study human NK cells in vivo. IL-15 receptor agonists may provide therapeutic tools to improve NK cell reconstitution after bone marrow transplants, enhance graft versus leukemia effects, and increase the pool of IL-15–responsive cells during immunotherapy strategies.
NK cells participate in host protection by eliminating cells with altered expression of self–MHC class I (MHC-I), which can result from viral infection or transformation (1). Although we are beginning to appreciate a role for viral and stress-induced ligands in NK cell activation, the best described regulatory mechanism of NK cell activity is the expression of inhibitory receptors for self–MHC-I ligands by mature NK cells with high cytotoxic potential. In man, killer Ig-like receptors (KIRs) recognizing the classical MHC-I molecules HLA-A, -B, or -C are expressed on the predominate peripheral NK cell (CD56loCD16+) subset, which possesses abundant intracellular perforin and granzymes and displays spontaneous cytotoxicity (1). In contrast, CD56hiCD16− NK cells rarely express KIRs, and because they are more prevalent in blood early after bone marrow transplant (2, 3), give rise to CD56loCD16+ NK cells when transferred into NOD/SCID mice (4), and have longer telomeres than CD56loCD16+ NK cells (5), it is likely that CD56hiCD16− NK cells are (or contain within this population) precursors of CD56loCD16+ NK cells; however, the tools to definitively prove this hypothesis are lacking.
Although inhibitory KIRs control reactivity of mature NK cells, their expression also influences the functional maturation of developing NK cells, as NK cells expressing at least one KIR-recognizing self–MHC-I have a lower threshold of activation and appear more functional than NK cells expressing no KIRs or those only expressing KIRs recognizing non-self–MHC-I ligands (6). In addition, patients lacking the transporter associated with antigen processing have dramatically reduced MHC-I surface expression and, consequently, hyporesponsive NK cells to MHC-I–deficient cells (7). KIR+ NK cells are present in these patients, indicating that normal MHC-I expression itself is not required for KIR expression (7). This phenomenon termed “licensing” or “disarming” has been better characterized in mice, and suggests a role for KIR–self–MHC-I interactions during human NK cell development. Given the importance of KIR expression in regulating NK cell function, knowledge of elements influencing KIR acquisition would improve our understanding and clinical approaches to diseases where KIR and HLA haplotypes influence susceptibility, progression, or outcome of autoimmune/inflammatory disease, cancer, hematopoietic grafts, and infections (8).
Because KIRs are expressed on mature CD56loCD16+ NK cells, factors that influence NK cell homeostasis could potentially influence KIR acquisition in vivo. An elegant series of mouse studies using gene targeting and bone marrow chimeras have revealed that NK cell development requires IL-15Rα–expressing cells to chaperone IL-15 to the surface, where it is bioactive and significantly more potent in inducing activation and proliferation of IL-15Rβγ–expressing cells via trans-presentation (9, 10). When not bound to IL-15Rα, IL-15 appears to have a minimal effect on NK cell homeostasis in vivo (11, 12). In humans, mutations in IL-15Rα have not been reported; however, NK cells are dramatically reduced in patients carrying mutations in the common γ chain (γc) cytokine receptor (used in IL-15, -7, -4, -9, -2, and -21 signal transduction), Jak3, or the shared IL-2/-15Rβ, whereas they are present in IL-7Rα–deficient patients, suggesting that IL-15 may regulate human NK cell development (13, 14).
In vivo studies of NK cells have been largely restricted to mice, and although this line of experimentation is valuable, some of this knowledge is not transferable to human NK cell biology. A clear example of this is NK cell development, where the kinetics, frequency, and phenotype are clearly different between species (1). An intermediate between mouse and human in vivo studies exists in the form of human immune system (HIS) mice. A recently developed HIS mouse model is the engraftment of newborn BALB/c Rag2−/−γc−/− mice with human hematopoietic stem cells (HSCs) from fetal liver or cord blood (15, 16). BALB/c Rag2−/−γc−/− HIS mice represent a practical HIS model with high human chimerism, most lymphocyte lineages generated, and adaptive immune responses occasionally evoked (15, 16).
In this study, using HIS BALB/c Rag2−/−γc−/− mice engrafted with fetal liver HSCs, we study the in vivo role of IL-15 trans-presentation in human NK cell homeostasis.
RESULTS AND DISCUSSION
Human hematopoietic chimerism and NK cell development in HIS mice
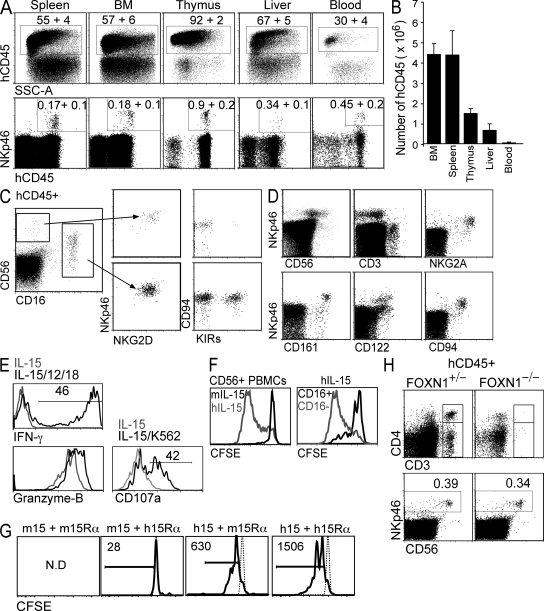
Human HSCs engrafted into newborn BALB/c Rag2−/−γc−/− mice develop into mature myeloid and lymphoid cells (15, 16). We used this approach to investigate human NK cell development in vivo. 8–12 wk after HSC engraftment, HIS mice displayed human hematopoietic chimerism in all organs analyzed, with human thymopoiesis, B lymphopoiesis, and myelopoiesis evident (Fig. 1, A and B, and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20082013/DC1). Using an NK cell–specific antibody (anti-NKp46), we identified human NK cells in all lymphoid organs of HIS mice, although at low frequencies, typically between 0.3 and 1.5% of human lymphocytes (Fig. 1 A). Both CD56hiCD16− and CD56loCD16+ NK cells subsets are present, with the majority of NK cells having the latter phenotype (Fig. 1 C). Interestingly, some CD16+ cells expressed CD56 at levels indistinguishable from non–NK cells. These CD56lo cells are NK cells, as they express NKp46, NKG2D, CD94, and, like their counterparts in man, KIRs, whereas CD56hiCD16− cells are rarely KIRs+ (Fig. 1 C). NKp46+ cells present a phenotype consistent with peripheral NK cells in man including expression of CD122 (IL-2Rβ), NKG2A, CD161, but not CD3 (Fig. 1 D). In vivo–generated human NK cells express high levels of intracellular granzyme B and IFN-γ when stimulated with IL-12 and -18 ex vivo, and they degranulate when co-cultured with K562 human leukemia cells as determined by expression of CD107a (Fig. 1 E).
Figure 1.
NK cells develop and populate various lymphoid tissues in HIS mice. (A) 8 wk after CD34+CD38− HSC engraftment, various organs from HIS mice were analyzed for human NK cell reconstitution by flow cytometry (human CD45; hCD45). FACS plots are representative. Values represent mean percentage ± SEM of eight mice. (B) The cellularity of the indicated organs was enumerated and the number of hCD45+ cells was determined based of flow cytometry data. Numbers of cells in bone marrow (BM) are per femur and in blood are per milliliter. Values represent mean ± SEM of eight mice. Human NK cells were analyzed in the thymus (C) and spleen or bone marrow (D) by flow cytometry using antibodies against the indicated human antigens. Events shown were pre-gated on hCD45+. FACS plots are representative of eight HIS mice, with a total of three different donor HSCs represented. (E) 5 × 104 CD56+ NK cells were purified from spleen and bone marrow of HIS mice and stimulated in vitro with IL-12 and -18 or 5 × 105 K562 CML cells in the presence of IL-15 for 18 h. Cells were stained with anti-CD107a or fixed, permeabilized, and stained with anti–IFN-γ or anti–granzyme B and analyzed by flow cytometry. (F) 2 × 105 CD56+ NK cells purified from human peripheral blood were labeled with CFSE and cultured in hIL-15 (gray line) or mIL-15 (black line) for 120 h. CFSE histograms of CD56loCD16+ (black line) and CD56hiCD16− (gray line) cultured in hIL-15 are also shown. (G) 104 CD56+ NK cells purified from human peripheral blood were labeled with CFSE and cultured with the indicated combinations of human (h) and mouse (m) IL-15 (15) and IL-15Rα-Fc (15Rα) for 72 h. Cells were analyzed by flow cytometry. Numbers indicate live cells (PI−) per well. N.D., not determined because there were no live cells. Histograms are representative of two individual experiments using different donor blood. (H) 8 wk after CD34+CD38− HSC engraftment, FOXN1+/− and FOXN1−/− (Nude) HIS mice were analyzed for human T and NK cell reconstitution by flow cytometry. FACS plots are gated on hCD45+ cells in spleen (top) and BM (bottom) and are representative of three mice of each genotype.
IL-15 is a pleiotropic cytokine essential for mouse NK cell development (10). Given the sparse number of human NK cells, we hypothesized that IL-15 availability in this HIS model might be suboptimal. HIS mice represent a hybrid human–mouse system where cytokine receptor compatibility between species may not exist. Specifically, although human IL-15 (hIL-15) induces survival and proliferation of mouse NK cells (17, 18), it is not clear whether the reverse is true. This is a critical question, as we expect most of the IL-15 in HIS mice to be mouse-derived given the low human myeloid (15) or absent epithelial/stromal cell chimerism (cells likely to be sources of IL-15) (10, 19). We found that human NK cells cultured in vitro with hIL-15 proliferated extensively (predominately the CD56hiCD16− subset); this was in contrast to those cultured in mouse IL-15 (mIL-15), which itself sufficiently induced proliferation of mouse NK cells (Fig. 1 F and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20082013/DC1). Given that mIL-15 is trans-presented in vivo, we investigated the effect of culturing human NK cells with low concentrations of IL-15 alone or IL-15 trans-presented by IL-15Rα-Fc of the same or alternate species. Irrespective of which species of IL-15Rα was used, mIL-15 failed to induce human NK cell proliferation, although some cells survived when mIL-15 was combined with hIL-15Rα-Fc (Fig. 1 G). In contrast, hIL-15Rα was clearly superior in inducing NK cell proliferation compared with mIL-15Rα when combined with hIL-15 (Fig. 1 G). Similarly, activated human, but not mouse, myeloid cells were able to induce human NK cell proliferation in vitro (Fig. S2). Last, human NK cells are observed in athymic HIS mice (Rag2−/−γc−/−FOXN1−/−), ruling out a major role for T cell–derived IL-2 driving NK cell development (Fig. 1 H).
Collectively, these data indicate that the few resident NK cells in HIS mice are dependent on the available hIL-15. Indeed, the number of human NK cells in the spleen of HIS mice could be further reduced after treatment with neutralizing antibody against human, but not mouse, IL-15 (Fig. S2). Furthermore, hIL-15Rα+ cells known to express IL-15 and support NK cell development, such as members of the myeloid lineage, are found in the bone marrow of HIS mice (unpublished data) (10, 15). Given this last point, it is possible that enhancing the chimerism of hIL-15/-15Rα–expressing cells will improve human NK cell development in HIS mice.
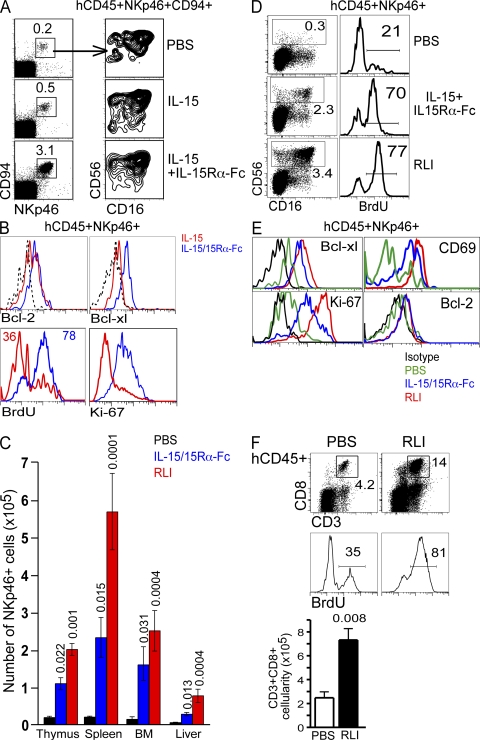
Given the likely dependence of human NK cells on hIL-15, we next treated HIS mice with the same concentration of hIL-15 alone or hIL-15 preincubated with hIL-15Rα-Fc to mimic IL-15 trans-presentation in vivo. Consistent with mouse studies (11, 12), we observed a significant increase in NK cell frequency when hIL-15 was complexed to IL-15Rα-Fc, but not when administered alone (Fig. 2 A). Increased NK lymphopoiesis by hIL-15+IL-15Rα-Fc compared with IL-15 alone was associated with increased Bcl-xL expression and cell proliferation, as demonstrated by BrdU uptake and Ki-67 expression (Fig. 2 B). These data indicate that IL-15R–mediated human NK cells responses are more readily evoked in vivo when IL-15 is complexed to IL-15Rα-Fc.
Figure 2.
Trans-presentation of hIL-15 promotes human NK cell homeostasis in vivo. (A) HIS mice were injected i.p. (every 5 d for 15 d) with hIL-15, IL-15+IL-15Rα-Fc, or PBS commencing 6 wk after reconstitution. 3 d after the last injection, bone marrow was analyzed for human NK cell reconstitution by flow cytometry using antibodies against the indicated human antigens. (B) HIS mice treated as in A were injected i.p. with 1 mg BrdU daily on the last 2 d before being killed. hCD45+NKp46+ cells from bone marrow were analyzed for intracellular proteins and incorporation of BrdU by flow cytometry. FACS plots are representative of two individual experiments using mice engrafted with and two different CD34+ HSC sources. (C) HIS mice were injected i.p. (once per week for 4 wk) with hIL-15+IL-15Rα-Fc, RLI, or PBS commencing 6 wk after reconstitution. 7 d after the last injection, mice were killed and thymus, spleen, liver, and bone marrow were analyzed for human NK cells by flow cytometry. Organ cellularities were counted and hCD45+NKp46+ cells were enumerated based on positive surface expression determined by flow cytometry. Data are mean ± SEM of five mice in each group generated from two different CD34+ HSC sources. P values are given when statistically significant. (D) NK cell subsets and BrdU incorporation in the bone marrow of HIS mice treated as in C were analyzed by flow cytometry. (E) hCD45+NKp46+ cells from bone marrow were analyzed for intracellular proteins and incorporation of BrdU by flow cytometry. For D and E, HIS mice were treated as in C; however, mice were killed 3 d after final treatment and were injected i.p. with 1 mg BrdU daily on the last 2 d before being killed. FACS plots are representative of two individual experiments of 3–5 mice per treatment group engrafted with two different CD34+ HSC sources. (F) CD8 T cells in the spleen of RLI-treated HIS mice were enumerated and analyzed for BrdU incorporation by flow cytometry. FACS plots are representative of four mice and gated on hCD45+ cells. Histograms are further gated on CD3+CD8+ cells. Cellularities are mean ± SEM of four mice.
We next compared two different IL-15R agonists, both mimicking IL-15 trans-presentation, hIL-15+IL-15Rα-Fc, and RLI (hIL-15 covalently linked to an hIL-15Rα extended sushi domain, but lacking any Fc fragment) (11, 12, 20). Four injections of 2.5 μg hIL-15R agonists (one per week) resulted in a significant increase in the number of NKp46+ cells in all organs (Fig. 2 C). Both these agonists markedly enhanced NK cell in vivo uptake of BrdU; expression of Bcl-xL, Ki-67, and CD69; and resulted in delayed apoptosis of NK cells when withdrawn from cytokines (Fig. 2, D and E, and not depicted), suggesting both proliferation and survival result from IL-15+IL-15Rα binding to NK cells in vivo. Human CD8 T cells were also significantly augmented in HIS mice and had an obvious increase in BrdU uptake after treatment with RLI (Fig. 2 F). Interestingly, RLI that activates both the IL-15Rβ/γ and IL-15Rα/β/γ was consistently more effective in vivo than noncovalent association of hIL-15+hIL-15Rα-Fc that activates IL-15Rβ/γ alone. This observation is in agreement with in vitro studies showing RLI functions more efficiently than the noncovalent associations of IL-15 + sushi domain or other soluble forms of IL-15R (20, 21). The evident effect of RLI compared with IL-15+IL-15Rα-Fc also indicates that the Fc protein does not contribute to the augmented NK lymphopoiesis and rules out any activation of human NK cells through CD16.
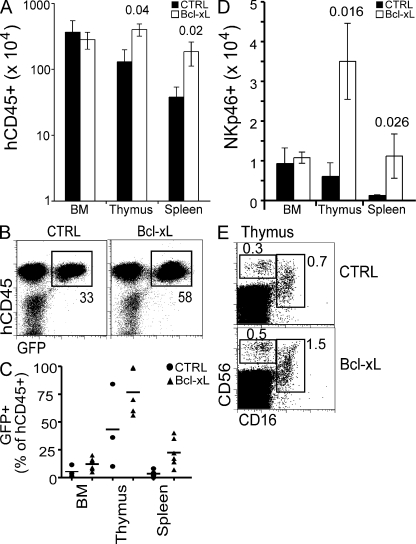
Although IL-15 is known to heighten NK cell cytotoxicity and induce proliferation, it also protects cells from apoptosis (the latter possible at lower concentrations) by suppressing proapoptotic Bim and elevating Bcl-2 family members such as Bcl-xL and Mcl-1 (18, 22, 23). A clear in vivo effect of RLI and IL-15+IL-15Rα-Fc was the up-regulation of Bcl-xL in NK cells (Fig. 2, B and E). We next asked if enhanced survival could improve NK cell reconstitution in the limiting hIL-15 environment of HIS mice. To address this, HSCs were infected with a bicistronic retrovirus encoding the pro-survival protein Bcl-xL and GFP (to detect infected cells) in vitro before engrafting newborn BALB/c Rag2−/−γc−/− mice. Ectopic expression of Bcl-xL in human HSCs resulted in a significant increase in thymocyte and splenocyte cellularity 8 wk after engraftment, with Bcl-xL–transduced cells (GFP+) representing a greater proportion of hCD45+ cells compared with control transduced cells in all organs (Fig. 3, A–C). NKp46+ cells were significantly increased in thymus and spleen of Bcl-xL HIS mice compared with control HIS mice; however, this appeared to be primarily a result of increased cellularity in these organs as the percentage of NK cells largely unchanged (Fig. 3, D and E). In addition, although a greater proportion of NK cells were GFP+ in Bcl-xL–infected mice compared with controls, no accumulation of Bcl-xL–expressing NK cells was observed among the most mature subset (CD56loCD16+). Lastly, the number of NK cells in HIS mice engrafted with Bcl-xL–infected HSCs, in terms of absolute numbers and fold difference compared with control, were substantially lower than mice treated with trans-presented IL-15. These findings indicate that IL-15/IL-15Rα effects extend beyond providing survival signals in promoting human NK cell development in vivo.
Figure 3.
Ectopic expression of Bcl-xL mildly augments human NK cell reconstitution in vivo. Human fetal liver HSCs were infected with retrovirus encoding hBcl-xL and GFP or GFP alone and used to generate HIS mice. (A) 8 wk after engraftment, thymus, spleen, and bone marrow cellularities were counted and CD45+ cells were enumerated based on surface expression as determined by flow cytometry. (B) GFP expression within hCD45+ thymocytes of HIS mice 8 wk after engraftment was detected by flow cytometry. (C) The percentages of GFP+ cells within the human graft were enumerated by flow cytometry. (D) NK cells were enumerated based on the data in A and surface expression hCD45 and NKp46 determined by flow cytometry. (E) NK cell subsets were analyzed by flow cytometry to detect surface expression of CD56 and CD16. FACS plots are pre-gated on hCD45+ cells and are representative of three to four mice in each group. Data in A and D are mean ± SEM of 3–6 mice in each group. P values are given when statistically significant.
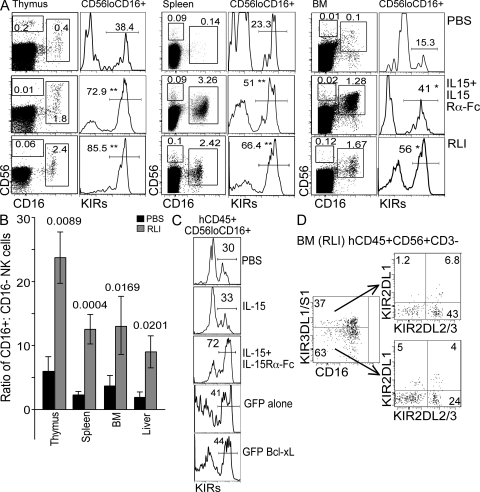
A consistent observation in all lymphoid organs after treatment with trans-presented IL-15 was the skewing of NK cell maturation toward the more differentiated CD56loCD16+ phenotype. In particular, we observed significant and specific increases in the frequency of CD16+ NK cells and the ratio of CD16+/CD16− NK cells 7 d after the final treatment (Fig. 4, A and B). This in vivo accumulation is consistent with a model where CD56loCD16+ NK cells represents the terminal stage of NK cell development and suggests that trans-presented IL-15 promotes this differentiation. CD16 expression is associated with human NK cell differentiation and increased cytotoxicity by means of increased intracellular effector granules and the ability to perform antibody-dependent cell cytotoxicity. IL-15 itself is known to enhance NK cell cytotoxicity via up-regulating effector molecules, such as IFN-γ, perforin, and granzymes (24). Strikingly, among the enhanced CD16+ population after IL-15 trans-presentation treatment, the percentage of NK cells expressing KIRs was also significantly elevated, resulting in a large increase in the KIR+ NK cell pool, an effect that is also more prominent with RLI (Fig. 4 A). The fraction of KIR+ NK cells after exogenous IL-15 trans-presentation was typically highest in the thymus and elevated compared with normal frequencies in human blood (typically 50–60%). This may result from greater IL-15 concentrations in our model compared with humans; however, the frequency of KIR+ NK cells in human thymus has not been reported using all existing commercial antibodies to KIRs. In contrast to IL-15 trans-presentation, no enhanced NK cell differentiation and induction of KIR expression was observed in HIS mice treated with IL-15 alone or in those engrafted with Bcl-xL–expressing HSCs (Fig. 4 C). Furthermore, the increase in KIR+ NK cells did not represent an expansion of one CD56loCD16+KIR+ NK clone, as CD56loCD16+ NK cells expressing a combination of 1–5 different KIR members were present in this population (Fig. 4 D).
Figure 4.
HIL-15R agonists promote NK cells differentiation in vivo. (A) NK cell maturation and KIR expression was analyzed in thymus, bone marrow, and spleen of HIS mice treated as in Fig. 2 C by flow cytometry for surface expression of CD56, CD16, and KIRs (KIR-2DL2/3/1/-2DS1/2/4/-3DL1/S1). HIS mice were killed 7 d after the last treatment. Histograms are gated on hCD45+CD56loCD16+ cells and are representative of five mice and two different CD34+ HSC sources. **, P < 0.01; *, P < 0.05. (B) The ratio of CD16+/CD16− NK cells (hCD45+NKp46+) in HIS mice treated with RLI and PBS as in Fig. 2 C were determined. Data are mean ± SEM of four mice in each group. P values are given when statistically significant. (C) Expression of KIRs (KIR-2DL2/3/1/-2DS1/2/4/-3DL1/S1) on CD56loCD16+ in HIS mice treated as in Fig. 2 A or engrafted with modified HSCs as in Fig. 3. FACS plots were pre-gated on hCD45+ and are representative of at least three mice in each group. (D) Expression of KIR2DL1/2/3 was analyzed on KIR3DL1/DS1+ or KIR3DL1/DS1— NK cells in the bone marrow of HIS mice treated with RLI as in Fig. 2 C. FACS plots are gated on hCD45+CD56+CD3− and are representative of three mice.
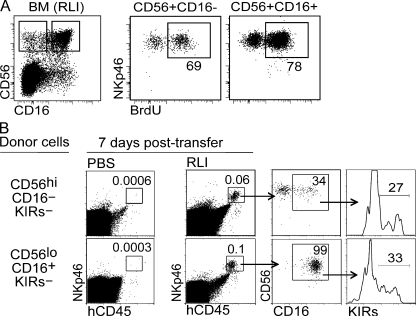
It is unlikely the accumulation of CD56loCD16+ NK cells results from a preferential expansion of this subset in response to hIL-15 trans-presentation as both CD56loCD16+ and CD56hiCD16− NK cell subsets had a similar level of incorporated BrdU during treatment (Fig. 5 A). Although one cannot distinguish between CD16+ NK cells that have incorporated BrdU and BrdU+ cells that have up-regulated CD16, it is most likely that the latter accounts for most of the BrdU+CD16+ NK cells as CD56loCD16+ NK cells are refractory to IL-15 stimulation in vitro (25, 26), especially when compared with CD56hiCD16− NK cells (Fig. 1 E). To test this hypothesis, we sorted and transferred either highly purified CD56hiCD16−KIRs− or CD56loCD16+KIRs− NK cells from fetal spleen into BALB/c Rag2−/−γc−/− in the presence or absence of RLI treatment. Although we were unable to recover the donor cells in mice receiving PBS alone, clear populations of donor cells were recovered from RLI-treated mice 7 d after transfer (Fig. 5 B). Consistent with our hypothesis, trans-presented IL-15 induced the differentiation of CD56hiCD16− NK cells, with between 34 and 40% of recovered NK cells becoming CD16+ (Fig. 5 B). In addition a fraction of the CD16+ NK cells also now expressed KIRs. Similarly, whereas CD56loCD16+ NK cells retained this phenotype in the presence of RLI after transfer, expression of KIRs were acquired by a fraction of these CD56loCD16+ NK cells that lacked KIR expression before transfer (Fig. 5 B). The absence of NK cells in non-RLI treated recipients clearly shows that the available mouse IL-15 is inadequate to support human NK cell survival and highlights the importance of a source of hIL-15 in HIS mice in promoting NK cell differentiation.
Figure 5.
IL-15-dependent development of CD16+KIR+ NK cells from CD16−KIR− precursors in vivo. (A) NK cell subsets in the bone marrow of RLI-treated HIS mice from Fig. 2 D were analyzed for BrdU uptake using flow cytometry. FACS plots are representative of two individual experiments of three to five mice per treatment group engrafted with two different CD34+ HSC sources. (B) CD56hiCD16−KIRs− or CD56loCD16+KIRs− NK cells were FACS sorted from fetal spleen (CD45+CD3−) and transferred intrahepatically into 1-wk-old sublethally irradiated Rag2−/−γc−/− recipients for 7 d. Mice were treated i.p. with 2.5 μg RLI or PBS on day 0 and 4 after transfer. Sorted KIRs− NK cells lacked KIR-2DL2/3/1/-2DS1/2/4/-3DL1/S1 expression. Data are representative of four mice per NK subset transferred and two mice per treatment group.
Binding of IL-15 to NK cells ultimately activates signaling pathways stemming from STAT5 phosphorylation, such as activation of NF-κB, induction of cyclin D, down-regulation of proapoptotic proteins, and up-regulation of Bcl-2 family members and cytolytic granules (18, 24, 27). Although it is clear how these signaling events promote cell division, survival, and effector functions, it is not known how these pathways regulate acquisition of KIRs. Coordination between granzyme/perforin induction and KIR expression would be one means to limit NK cell activity during differentiation, and whereas most NK cells express other inhibitory receptors for MHC-I (CD94/NKG2A and LILR) throughout development, it appears acquisition of KIRs is a late event and likely unique compared with other forms of MHC-I–mediated inhibition. Collectively, these data provide the first in vivo demonstration that trans-presented IL-15 is essential for NK cell survival and subsequent differentiation and that KIR expression on mature NK cells is dynamically regulated by IL-15 signaling.
Concluding remarks
Mouse studies show that IL-15 functions as a membrane-bound cytokine that can only be present at the cell surface and support NK cell development when bound to IL-15Rα on the same cell (9, 10). The clear effect of hIL-15Rα to enhance hIL-15 activity in vivo suggests this is also likely in man. Our findings using a novel HIS mouse approach demonstrates that hIL-15 trans-presentation is necessary to promote human NK cell development and differentiation in vivo. The failure to observe accumulation of CD16+KIR+ NK cells in HIS mice with HSCs ectopically expressing Bcl-xL suggests that NK cell survival alone is not sufficient to promote differentiation and a source of hIL-15 is essential for this process.
Factors regulating KIR expression on developing NK cells in vivo are undefined. Our study provides the first evidence that trans-presented IL-15 induces KIR expression on CD56loCD16+ NK cells in vivo and suggests that KIRs are expressed after CD16 and that acquisition of KIRs represents a further step in NK cell differentiation. Interestingly, expression of human self–MHC-I on a fraction of the hematopoietic cells in HIS mice appears sufficient in generating phenotypically mature KIR+ NK cells. Our findings suggest use of hIL-15R agonists in vivo may augment human NK cell differentiation, and subsequently functionality, and could benefit patients with underrepresented populations of mature cytotoxic KIR+ NK cells and improve NK cell– or CD8 T cell–based cancer immunotherapy strategies. The efficiency of IL-15R agonists, particularly RLI, in augmenting human NK cell development in HIS mice will enable us to more readily dissect the role of IL-15-dependent lymphocytes (NK cell, memory CD8 T cell, NK T cell, and γ/δ T cell) responses to human pathogens and disease in vivo. Given obvious cross talk between innate and adaptive immune cells, having robust reconstitution of IL-15–dependent cells in vivo improves the accuracy and application of HIS mice for studying human immune responses. Collectively, our results define an essential role for IL-15 in human NK cell development in vivo and demonstrate the efficacy of IL-15–IL-15Rα complexes in promoting human NK cell homeostasis, suggesting hIL-15 is likely bound and trans-presented by IL-15Rα–expressing cells in man.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Harlan. Rag2−/−γc−/− mice were backcrossed onto the BALB/c background and BALB/c Rag2−/−γc−/− mice on a Nude background were bred and maintained in isolators with autoclaved food and water under a protocol approved by the AMC-UVA. Mice with a HIS were generated as previously described (15, 16). In brief, newborn (3–5-d-old) Rag2−/−γc−/− mice received sublethal (3.3 Gy) total body irradiation from a Cs source, and were injected intrahepatically with 105 sorted CD34+CD38− or 5 × 105 CD34+ human fetal liver cells. All manipulations of HIS mice were performed under laminar flow. Mouse experiments were approved by an institutional committee at the Institut Pasteur and validated by the French Ministry of Agriculture.
In vivo treatments.
HIS mice were injected i.p. with 100 μl of hIL-15 (2.5 μg) and hIL-15Rα-Fc (7.5 μg) + hIL-15 (2.5 μg) both from R&D Systems, 2.5 μg RLI (20), or PBS alone, commencing at a minimum of 6 wk after reconstitution. 100 μl of 10 mg/ml of BrdU from BrdU flow kit (BD) was injected i.p. daily 2 d before sacrificing mice. For NK cell transfer assays, 3 × 104 CD56hiCD16−KIRs− or 2 × 105 CD56loCD16+KIRs− NK cells were sorted from human CD45+CD3− fetal splenocytes (15-wk gestation) and transferred intrheaptically into 1-wk-old sublethally irradiated BALB/c Rag2−/−γc−/− mice. A cocktail of KIR antibodies recognizing KIR-2DL2/3/1/-2DS1/2/4/-3DL1/S1 was used. Recipients were then treated i.p. with PBS or 2.5 μg of RLI on day 0 and 4 after transfer. IL-15 in vivo neutralization was performed by i.p. administration of 50 μg of goat anti–hIL-15 or 25 μg of goat anti–mouse IL-15 (both from R&D Systems) every second day for 7 d.
Flow cytometry analysis for cell surface and intracellular markers.
Cell suspensions were labeled with mAb against the following human cell surface markers: CD3 (SK7), CD4 (SK3), CD34 (581), CD8 (SK1), CD19 (HIB19), CD10 (HI10a), CD38 (HB7), NKG2D (1D11), NKp46 (9E2), CD16 (3G8), CD161 (DX12), CD56 (B159), HLA-DR (L243), HLA-A/B/C (G46-2.6), CD117 (YB5.B8), IgD (IA6-2), IgM (G20-127), CD14 (M5E2), CD122 (Mik-β3), TCR-α/β (T10B9.1A-31), CD127 (hIL-7R-M21), CD11c (B-ly6), CD7 (M-T701), CD107a (1D4B), CD45 (2D1), CD69 (L78), Bcl-2 (Bcl-2/100), IFN-γ (XMG1.2), BrdU (B44), and Ki67 (B56) from BD; KIR2DL2/L3 (DX27), KIR3DL1 (DX9), CD94 (DX22), NKp44 (p44-8), CD27 (O323), CD62L (DREG-56), and NKp30 (P30-15) from BioLegend; KIR2DS4 (FES172), KIR2DL1/DS1 (EB6B), KIR2DL2/L3/DS2 (GL183), KIR3DL1/DS1 (Z27.3.7), Bcl-xL (7B2.5), and CD159a (NKG2A; Z199) from Beckman Coulter; granzyme B (GB11) from Invitrogen; and CD11b (ICRF44), CD25 (BC96), CD116 (4H1), CD83 (HB15e), anti–mouse NK1.1, CD11b, CD11c, F480, and DX5 from eBioscience. Intracellular staining was performed after fixation and permeabilization of the cellular suspensions using Perm/Wash and Cytofix/Cytoperm reagents from BD according to the manufacturer's instruction. For BrdU detection, cells were incubated for 1 h at 37°C with 30 μg DNase from BrdU flow kits (BD). All washings and reagent dilutions were done with PBS containing 2% FCS. All acquisitions were performed using LSRII, Canto 1, or Canto 2 cytometers, cell sorting was performed using FACSAria, and all machines were interfaced to the FACSDiva software (BD).
Cell preparation.
Human fetal liver was obtained from elective abortions, with gestational age ranging from 14 to 20 wk. Experiments using human fetal liver cells were approved by the Medical and Ethical Committees at the Institut Pasteur and AMC-UvA and performed in full compliance with French law. Single-cell suspensions of fetal material were achieved by mechanical disruption using a Stomacher Biomaster laboratory system (Seward). Magnetic enrichment of CD34+ cells (>98% pure) was performed by using the CD34 Progenitor Cell Isolation kit (Miltenyi Biotech, Auburn, CA), after preparation of single-cell suspension and isolation of mononuclear cells by density gradient centrifugation over Ficoll-Hypaque (Nycomed Pharma). Cell suspensions were prepared in RPMI medium with 2% FCS. Single-cell suspensions of mouse organs were prepared as previously described (17).
In vitro assays.
Human NK cells were purified from donor blood buffy coat prepared by density gradient centrifugation over Ficoll-Hypaque (Nycomed Pharma) using anti-CD56 magnetic beads (Miltenyi Biotech). C57BL/6 splenic NK cells were purified by anti-DX5 magnetic beads (Miltenyi Biotech). Purified cells were loaded with 5 μM CFSE (Invitrogen) and cultured at 2 × 105 cells/ml in RPMI with 10% FCS and 10 ng/ml rhIL-15 (rhIL-15; R&D Systems) or 30 ng/ml rmIL-15 (Peprotech) for 3 or 5 d. Alternatively, 104 CD56+ NK cells purified from human peripheral blood were labeled with CFSE and cultured for 72 h in combinations of human or mouse IL-15 that had been preincubated with human or mouse IL-15Rα-Fc for 1 h at 4°C. The final concentration of IL-15 and IL-15Rα-Fc were 5 and 20 ng/ml, respectively. In vitro restimulation of HIS-derived NK cells was performed using CD56+ NK cells purified by magnetic beads (Miltenyi Biotech) from a cell suspension of spleen and bone marrow from seven HIS mice pooled together. NK cells were cultured at 2.5 × 105 cells/ml in RPMI supplemented with 10% FCS, rhIL-15 (5 ng/ml), and either rhIL-12 (5 ng/ml) + rhIL-18 (20 ng/ml; R & D Systems) or 2.5 × 106 K562 cells/ml (American Type Culture Collection) for 18 h, with brefeldin A (Sigma-Aldrich) added for the last 4 h of culture.
Retroviral Bcl-xL expression.
Human fetal liver cells were prepared and modified by retroviral transduction as follows. The sorted CD34+CD38− fetal liver cells were cultured overnight in IMDM (Invitrogen) supplemented with Yssel's medium, 5% normal human serum, 20 ng/ml human stem cell factor, 20 ng/ml human thrombopoietin, and 20 ng/ml human IL-7 (PeproTech). The next day, cells were incubated for 6–8 h with control LZRS IRES-GFP or LZRS Bcl-xL-IRES-GFP virus supernatant in fibronectin-coated plates (30 μg/ml; Takara Biomedicals). The cell bulk was then inoculated intrahepatically to the newborn recipients. The cDNA sequence encoding Bcl-xL, initially provided by the laboratory of J.G. Collard (The Netherlands Cancer Institute, Amsterdam, Netherlands) was inserted into the multiple cloning sites of LZRS vector upstream of an internal ribosomal entry site and enhanced GFP (28). Control vectors were empty LZRS IRES. Retroviral supernatants were produced as previously described (29) using the 293T-based Phoenix packaging cell line (30).
Online supplemental material.
Fig. S1 shows lymphocyte reconstitution in HIS mice. Fig. S2 shows in vitro NK cell cultures and in vivo IL-15 neutralization. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082013/DC1.
Supplementary Material
Acknowledgments
We acknowledge the Bloemenhove Clinic (Heemstede, The Netherlands) for providing fetal tissues. We would like to thank Allison Bordack, Dr. Jean-Jacques Mention, Dr. Ferenc Scheeren, Joran Volmer, and Jenny Meerding for reagents and technical assistance and Prof. Andreas Strasser for critiques on the manuscript.
This work is supported by grants from Institut Pasteur, Institut National de la Santé et de la Recherche Médicale, Ligue National Contre le Cancer, National Health and Medical Research Council of Australia, The Menzies Foundation, and a Grand Challenges in Global Health grant from the Bill and Melinda Gates Foundation. Authors from the Institut Pasteur and Academic Medical Center are part of the Human Vaccine Consortium “Grand Challenges in Global Health: Devise Reliable Testing Sysytems for New Vaccines:” (http://www.hv-consortium.org).
The authors have no conflicting financial interest.
H. Spits's present address is Genentech, Inc., South San Francisco, CA 94080.
References
- 1.Huntington, N.D., C.A. Vosshenrich, and J.P. Di Santo. 2007. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7:703–714. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs, R., M. Stoll, G. Stratmann, R. Leo, H. Link, and R.E. Schmidt. 1992. CD16- CD56+ natural killer cells after bone marrow transplantation. Blood. 79:3239–3244. [PubMed] [Google Scholar]
- 3.Gottschalk, L.R., R.A. Bray, H. Kaizer, and H.M. Gebel. 1990. Two populations of CD56 (Leu-19)+/CD16+ cells in bone marrow transplant recipients. Bone Marrow Transplant. 5:259–264. [PubMed] [Google Scholar]
- 4.Chan, A., D.L. Hong, A. Atzberger, S. Kollnberger, A.D. Filer, C.D. Buckley, A. McMichael, T. Enver, and P. Bowness. 2007. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J. Immunol. 179:89–94. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani, C., K. Juelke, M. Falco, B. Morandi, A. D'Agostino, R. Costa, G. Ratto, G. Forte, P. Carrega, G. Lui, et al. 2007. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 178:4947–4955. [DOI] [PubMed] [Google Scholar]
- 6.Anfossi, N., P. Andre, S. Guia, C.S. Falk, S. Roetynck, C.A. Stewart, V. Breso, C. Frassati, D. Reviron, D. Middleton, et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 25:331–342. [DOI] [PubMed] [Google Scholar]
- 7.de la Salle, H., D. Hanau, D. Fricker, A. Urlacher, A. Kelly, J. Salamero, S.H. Powis, L. Donato, H. Bausinger, M. Laforet, et al. 1994. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 265:237–241. [DOI] [PubMed] [Google Scholar]
- 8.Carrington, M., and M.P. Martin. 2006. The impact of variation at the KIR gene cluster on human disease. Curr. Top. Microbiol. Immunol. 298:225–257. [DOI] [PubMed] [Google Scholar]
- 9.Mortier, E., T. Woo, R. Advincula, S. Gozalo, and A. Ma. 2008. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657–679. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein, M.P., M. Kovar, J.F. Purton, J.H. Cho, O. Boyman, C.D. Surh, and J. Sprent. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc. Natl. Acad. Sci. USA. 103:9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoklasek, T.A., K.S. Schluns, and L. Lefrancois. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley, R.H. 2004. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu. Rev. Immunol. 22:625–655. [DOI] [PubMed] [Google Scholar]
- 14.Gilmour, K.C., H. Fujii, T. Cranston, E.G. Davies, C. Kinnon, and H.B. Gaspar. 2001. Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood. 98:877–879. [DOI] [PubMed] [Google Scholar]
- 15.Gimeno, R., K. Weijer, A. Voordouw, C.H. Uittenbogaart, N. Legrand, N.L. Alves, E. Wijnands, B. Blom, and H. Spits. 2004. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 104:3886–3893. [DOI] [PubMed] [Google Scholar]
- 16.Traggiai, E., L. Chicha, L. Mazzucchelli, L. Bronz, J.C. Piffaretti, A. Lanzavecchia, and M.G. Manz. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 304:104–107. [DOI] [PubMed] [Google Scholar]
- 17.Huntington, N.D., Y. Xu, S.L. Nutt, and D.M. Tarlinton. 2005. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J. Exp. Med. 201:1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntington, N.D., H. Puthalakath, P. Gunn, E. Naik, E.M. Michalak, M.J. Smyth, H. Tabarias, M.A. Degli-Esposti, G. Dewson, S.N. Willis, et al. 2007. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 8:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehniger, T.A., and M.A. Caligiuri. 2001. Interleukin 15: biology and relevance to human disease. Blood. 97:14–32. [DOI] [PubMed] [Google Scholar]
- 20.Mortier, E., A. Quemener, P. Vusio, I. Lorenzen, Y. Boublik, J. Grotzinger, A. Plet, and Y. Jacques. 2006. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J. Biol. Chem. 281:1612–1619. [DOI] [PubMed] [Google Scholar]
- 21.Bouchaud, G., L. Garrigue-Antar, V. Sole, A. Quemener, Y. Boublik, E. Mortier, H. Perdreau, Y. Jacques, and A. Plet. 2008. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Ralpha. J. Mol. Biol. 382:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Carson, W.E., T.A. Fehniger, S. Haldar, K. Eckhert, M.J. Lindemann, C.F. Lai, C.M. Croce, H. Baumann, and M.A. Caligiuri. 1997. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng, X., Y. Wang, H. Wei, B. Ling, R. Sun, and Z. Tian. 2008. Bcl-xL is associated with the anti-apoptotic effect of IL-15 on the survival of CD56(dim) natural killer cells. Mol. Immunol. 45:2559–2569. [DOI] [PubMed] [Google Scholar]
- 24.Fehniger, T.A., S.F. Cai, X. Cao, A.J. Bredemeyer, R.M. Presti, A.R. French, and T.J. Ley. 2007. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 26:798–811. [DOI] [PubMed] [Google Scholar]
- 25.Carson, W.E., J.G. Giri, M.J. Lindemann, M.L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M.A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooley, S., F. Xiao, M. Pitt, M. Gleason, V. McCullar, T.L. Bergemann, K.L. McQueen, L.A. Guethlein, P. Parham, and J.S. Miller. 2007. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 110:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giron-Michel, J., M. Giuliani, M. Fogli, D. Brouty-Boye, S. Ferrini, F. Baychelier, P. Eid, C. Lebousse-Kerdiles, D. Durali, R. Biassoni, et al. 2005. Membrane-bound and soluble IL-15/IL-15Ralpha complexes display differential signaling and functions on human hematopoietic progenitors. Blood. 106:2302–2310. [DOI] [PubMed] [Google Scholar]
- 28.Jaleco, A.C., A.P. Stegmann, M.H. Heemskerk, F. Couwenberg, A.Q. Bakker, K. Weijer, and H. Spits. 1999. Genetic modification of human B-cell development: B-cell development is inhibited by the dominant negative helix loop helix factor Id3. Blood. 94:2637–2646. [PubMed] [Google Scholar]
- 29.Heemskerk, M.H., B. Blom, G. Nolan, A.P. Stegmann, A.Q. Bakker, K. Weijer, P.C. Res, and H. Spits. 1997. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J. Exp. Med. 186:1597–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsella, T.M., and G.P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405–1413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.