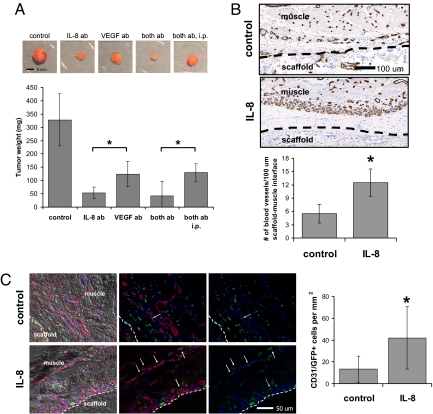
Fig. 5.
Systemic and local effects of IL-8 on tumor vascularization and progression in vivo. (A) Antiangiogenic therapy using delivery of antibodies blocking IL-8 and VEGF either individually (IL-8 ab, VEGF ab) or simultaneously (both ab, both ab i.p.) inhibited tumor growth compared with the no-antibody control condition. Localized delivery of neutralizing IL-8 antibody (IL-8 ab) inhibited tumor progression more significantly compared with delivery of VEGF antibody (VEGF ab) (P < 0.05) and localized delivery of a regimen that consisted of both neutralizing IL-8 and neutralizing VEGF antibody (both ab) inhibited tumor formation more extensively relative to the same therapy systemically applied via i.p. injection (both ab i.p.) (P < 0.05). (B) Localized and sustained delivery of IL-8 from polymeric scaffolds as a mimic of IL-8 secretion from tumors (IL-8) resulted in increased blood vessel formation at the muscle–scaffold interface relative to implantation of blank control scaffolds (control) as determined by image analysis of histological cross-sections stained for the endothelial cell marker CD31 (P < 0.05). Inside the scaffold, blood vessel density was similar in both the control and IL-8 condition (data not shown). Dashed lines indicate the muscle–scaffold boundary. (C) Localized IL-8 release increased recruitment of bone marrow-derived cells (green) to the vasculature (red). IL-8 delivery from polymer scaffolds (IL-8) in mice transplanted with EGFP bone marrow led to more cells that costained for CD31 and EGFP, compared with mice transplanted with blank control scaffolds (control) (P < 0.05) as analyzed by coimmunofluorescence of cryosections (see Fig. S9 for confirmation of colocalization of staining). Arrows and dashed lines indicate double-labeled cells and the muscle–scaffold boundary, respectively.