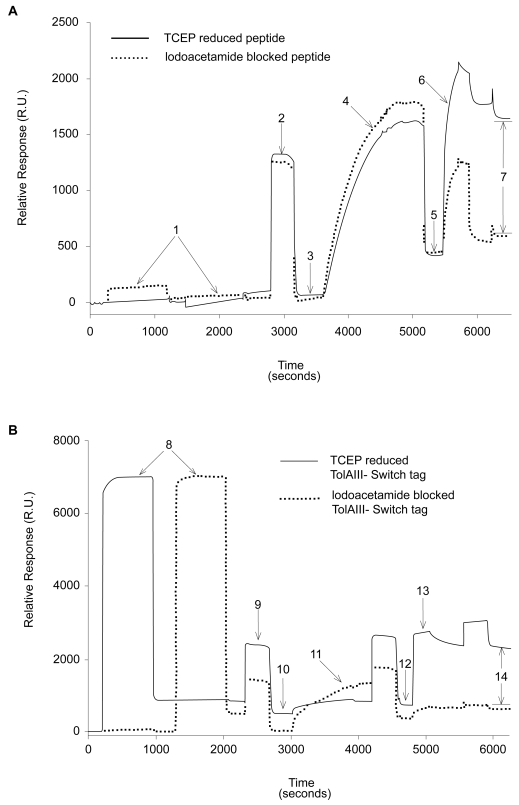
Figure 2.
SPR traces showing differential binding of reduced versus blocked FLAG-ST (A) and FLAG-TolAIII-ST (B) protein to gold surfaces. A buffer of 200 mM NaCl, 0.5 mM EDTA and 20 mM Tris pH 8 was used throughout. 25 μl injections of 0.1 mg/ml peptide or protein solution plus 0.5 mM TCEP at 2 μl/min were used (points 1 and 8). A 25 μl wash of running buffer plus 1% SDS at 5 μl/min (points 2 and 9) removed nonspecifically bound material, leaving immobilized molecules on the surface (3 and 10). 25 μl of 0.5 mM PEG-Thiol solution in running buffer was then injected at 2 μl/min and the surface again washed with 1% SDS as before. This led to a mass increase of around 350 R.U. (5 and 12). To ensure that the PEG-thiol had not displaced protein, 20 μl of 0.1 mg/ml anti-FLAG antibody solution was injected at 5 μl/min (6 and 13), followed by a wash of 25 μl 0.1% SDS solution. The amounts of specifically bound antibody (points 7 and 14) was found to be 1105 R.U. on the peptide surface and 1315 R.U. on the protein surface.
Abbreviations: SDS, sodium dodecyl sulfate; SPR, surface plasmon resonance; ST, switch tag peptide; TCEP, tris(2-carboxyethyl)phosphine.