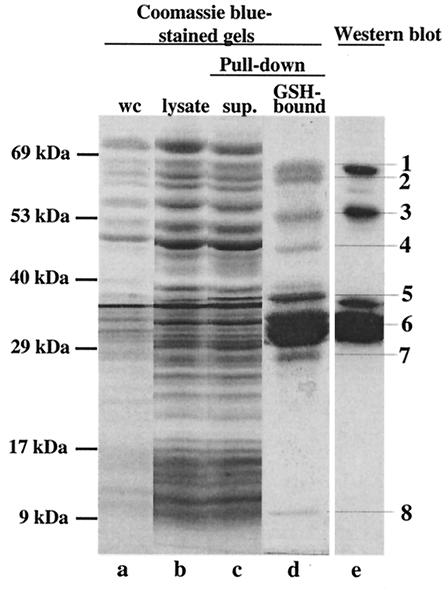
FIG. 2.
Pull-down assay to isolate putative binding partners of SopE. A SopE-GST fusion protein (pM113) was expressed in Salmonella serovar Typhimurium M566 (ΔsopB ΔsopE ΔsopE2 ΔsipA) (see Materials and Methods). The bacterial lysate (25 ml) was incubated with 200 μl of GSH-Sepharose beads. Samples from different purification steps were analyzed on a discontinuous SDS gel and were detected by staining with Coomassie brilliant blue (lanes a to d). Proteins bound to GSH-Sepharose were analyzed on a discontinuous 10% SDS-15% SDS gel and by Western blotting by using a mixture of anti-SopE and anti-GST antibodies (lane e). Lane a, 100 μl of a whole culture (wc) before harvesting of the cells; lane b, 25 μl of cleared cell lysate after lysis with a French pressure cell; lane c, 25 μl of supernatant after incubation of the cell lysate with GSH-Sepharose (sup.); lane d, SDS-PAGE analysis of proteins bound to 100 μl of GSH-Sepharose beads; lane e, Western blot analysis of 5% of the sample loaded in lane d performed with anti-SopE antiserum and an anti-GST antibody.