Table 1.
Facile N-Arylation of Aromatic Aminesa
| entry | amine | silylaryl triflate (equiv) |
CsF (equiv) |
time (h) |
product | % isolated yield |
|---|---|---|---|---|---|---|
| 1 | 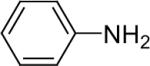 |
1a (1.1) | 2.0 | 10 | 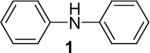 |
92 |
| 2 | 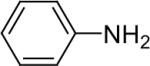 |
1b (1.1) | 2.0 | 10 | 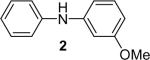 |
94 |
| 3 | 1a (1.1) | 2.0 | 10 | 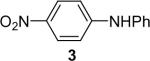 |
85 | |
| 4 | 1a (1.1) | 2.0 | 10 | 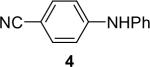 |
90 | |
| 5 | 1a (1.1) | 2.0 | 10 | 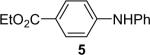 |
94 | |
| 6 | 1a (1.1) | 2.0 | 10 | 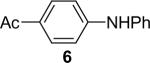 |
94 | |
| 7 | 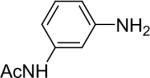 |
1a (1.1) | 2.0 | 10 | 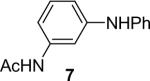 |
95 |
| 8 | 1a (1.1) | 2.0 | 10 | 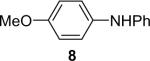 |
89b | |
| 9 | 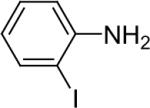 |
1a (1.1) | 2.0 | 10 | 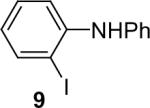 |
91 |
| 10 | 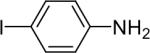 |
1a (1.1) | 2.0 | 10 | 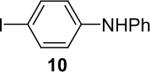 |
92 |
| 11 | 1a (1.1) | 2.0 | 10 | 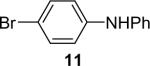 |
91 | |
| 12 | 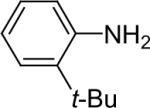 |
1a (1.1) | 2.0 | 10 | 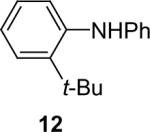 |
77 |
| 13 | 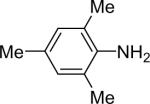 |
1a (1.1) | 2.0 | 10 | 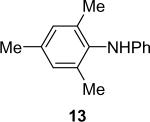 |
90 |
| 14 | 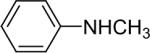 |
1a (1.1) | 2.0 | 5 | 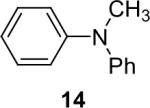 |
96 |
| 15 | 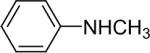 |
1c (1.1) | 2.0 | 10 | 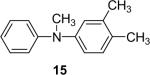 |
97 |
| 16 | 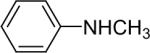 |
1d (1.1) | 2.0 | 5 | 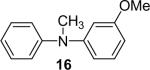 |
94 (1:1) |
| 17 | 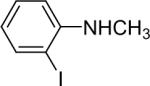 |
1a (1.1) | 2.0 | 5 | 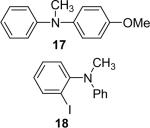 |
97 |
| 18 | 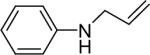 |
1a (1.1) | 2.0 | 5 | 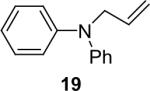 |
97 |
| 19 | 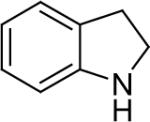 |
1a (1.1) | 2.0 | 5 | 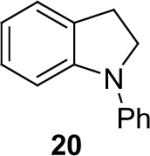 |
97 |
| 20 | 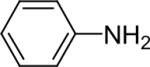 |
1a (2.4) | 4.0 | 72 | 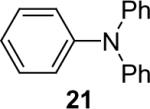 |
98 |
| 21 | 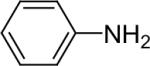 |
1b (2.4) | 4.0 | 72 | 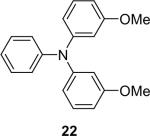 |
94 |
| 22 | 1c (2.4) | 4.0 | 72 | 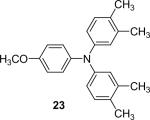 |
93 | |
| 23 | 1a (2.4) | 4.0 | 72 | 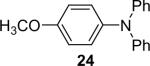 |
100 | |
| 24 | 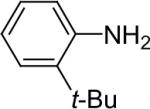 |
1a (2.4) | 4.0 | 72 | 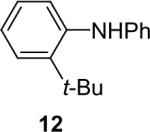 |
81c |
| 25 | 1a (2.4) | 4.0 | 72 | 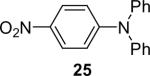 |
55d | |
| 26 | 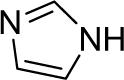 |
1a (1.2) | 2.0 | 24 | 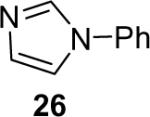 |
76 |
| 27 | 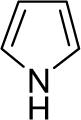 |
1a (3.6) | 6.0 | 72 | 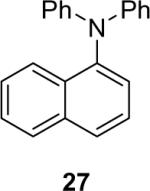 |
81 |
| 28 | 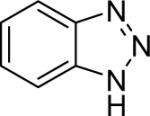 |
1a (1.2) | 2.0 | 24 | 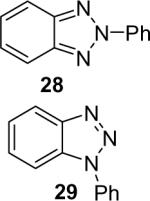 |
92 (1 : 3.5) |
All reactions were run under the following reaction conditions, unless otherwise specified: 0.25 mmol of amine and the indicated amount of silylaryl triflate and CsF were stirred in 4 mL of MeCN at room temperature.
A 4% yield of the diarylated product was obtained.
Only monoarylated product was obtained.
A 40% yield of monoarylated product was obtained.
