Abstract
There has been growing interest in the use of grafts in pelvic reconstructive surgery. This article will address available graft materials and assess their clinical efficacy and safety. We conducted a Pubmed MEDLINE literature search for full-length English text studies with follow-up periods of at least one year. There are many reports on synthetic and biological graft materials; the majority are not well-designed, have short-term follow-up, small sample sizes, and poor outcome assessment. The use of non-absorbable synthetic grafts may offer excellent anatomical cure rates. However, it is associated with a high incidence of graft-related complications, including healing abnormalities and adverse bladder, bowel, and sexual function effects. These complications can be decreased with absorbable synthetic meshes, but efficacy is lower compared to non-absorbable ones. There is insufficient evidence in favor of biological grafts. In conclusion, based on current knowledge, routine application of grafts in pelvic reconstruction is not recommended. It is preferred that graft utilization be individualized, with close monitoring for complications.
Keywords: Grafts, pelvic reconstructive surgery
INTRODUCTION
Pelvic organ prolapse is a common disorder that affects urinary, bowel, and sexual functions in women. The lifetime risk of surgery for pelvic organ prolapse and urinary incontinence is estimated to be 11%, with a 29% rate of reoperation.1 Failure rates increase with subsequent attempts at surgical correction. Suboptimal long-term outcomes reported with traditional anterior and posterior colporrhaphy have led to growing interest in grafts. Graft interposition helps reduce failure rates from plication of attenuated tissue and from lack of identifying all existent defects. Furthermore, readily available synthetic (mesh) and biological (allograft or xenograft) products may obviate the need for separate tissue harvesting and patient repositioning for autologous grafts, and thus reduce the required operating time.
After successful experiences with pubovaginal sling, tension-free vaginal tape, and abdominal sacrocolpopexy, various trials using grafts have been performed in vaginal pelvic reconstructions even to the extent of multi-compartmental pelvic floor defects with techniques using total vaginal mesh.2-4
Recently, a web-based survey was administered to American Urogynecologic Society members via email to evaluate attitudes and practice patterns regarding use of synthetic mesh in vaginal pelvic reconstructive surgery. Interestingly, among responders, those not fellowship-trained were 2.5 times more likely to use mesh than those trained (95% confidence interval 1.3-4.9, p=0.008).5
The main problem with the use of grafts in vaginal pelvic reconstructive surgery is the lack of efficacy and safety evidence. Clinical data are mostly derived from experiences in reconstructive surgery on ventral hernias, ophthalmology, and orthopedics. Although graft applications proved to be low risk in certain vaginal procedures, such as pubovaginal sling and tension-free vaginal tape, much more graft material is required for pelvic reconstructions. It is therefore expected that healing problems, such as extrusion, erosion, and rejection, will occur more frequently.
In this article, the general properties of available graft materials for pelvic reconstruction will be addressed, and their clinical efficacy and safety assessed based on results of the literature review.
GRAFT MATERIALS AVAILABLE FOR PELVIC RECONSTRUCTION
Graft materials are either synthetic or biological. Synthetic grafts are classified into absorbable and non-absorbable types, and subclassified into 4 groups based on physical properties. Biological grafts are classified into 3 groups, including autologous (rectus fascia, fascia lata, vaginal mucosa), allograft (cadaveric fascia lata, dermis), and xenograft (porcine dermis, small intestinal submucosa).
Synthetic grafts (Mesh)
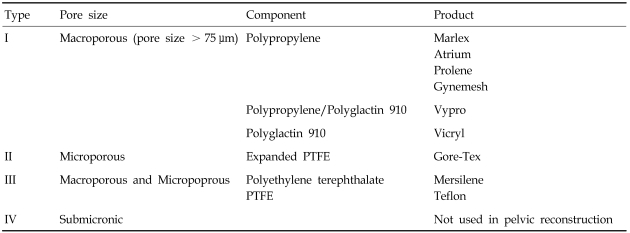
Mesh differs in several physical properties, including the type of filament (monofilamentous versus multifilamentous), pore size (macroporous (>75 microns) versus microporous (<10 microns)), porosity (defined as the difference between total fabric area and area covered by fabric), weight, architecture (knitted versus woven), and flexibility.
With regard to infection, mesh pore size is important because bacteria are, on average, less than 1 micron, whereas macrophages and neutrophilic granulocytes are larger than 10.6 Therefore, multifilamentous mesh, with interstices less than 10 microns, is prone to cause infection and requires removal in such cases. Ingrowth of fibrous tissue, a measure of graft success as scaffold, and flexibility or stiffness also depend on pore size.7,8 With larger pores, flexibility increases.9 Amid classified available biomaterials into 4 types by pore size.6 Mesh weight also relates to flexibility, and in animal studies, lighter materials have less contracture and folding during healing.10 Woven mesh is reported to have to a high incidence of healing problems.11
Table 1 shows several mesh types used for pelvic reconstruction, based on Amid's classification system. Type 1 mesh has been the preferred type, but in spite of excellent anatomical cure rates with mesh application, a high incidence of healing abnormalities and deteriorations in bladder, bowel, and sexual functions remain to be resolved.
Table 1.
Summary of Mesh Used for Pelvic Reconstruction
PTFE, polytetrafluoroethylene.
Biological grafts
The high incidence of complications associated with synthetic materials has led to the use of biological grafts. Autologous fascia may be an attractive material in terms of cost-effectiveness, availability, and biocompatibility. It also minimizes tissue property changes seen in processed versus fresh tissue specimens, and eliminates risk of donor viral transmission. Grafts are harvested either from the rectus fascia or from outer thigh fascia lata. Use of rectus fascia requires a larger abdominal incision, with greater potential surgical morbidity and recovery time. Tissue quality may also be compromised in women with inherent tissue weakness.12,13 Although fascia lata can be harvested through a small incision, with thicker and stronger tissues obtained, it requires patient repositioning for a second operative site and also has potential morbidity, including hematoma and seroma formation, incisional infection, and postoperative thigh pain.14 The vaginal wall patch may eliminate such problems but is not as strong as synthetic or fascial grafts.15 Therefore, autologous grafts have rarely been used in pelvic reconstruction.
The advantage of allografts or xenografts is avoidance of the time and morbidity of fascial harvest with the achievement of greater biocompatibility and lower erosion risk compared to synthetics. Cadaveric fascia lata/dermis and porcine dermis are commonly used as biological materials for pelvic reconstruction. However, use of these materials causes several concerns, such as durability, prion or virus transmission, or residual antigenicity causing graft versus host reactions.
In an experimental investigation of time-dependent changes in tensile strength, stiffness, shrinkage, and distortion among cadaveric, porcine dermis, porcine small intestine submucosa, polypropylene mesh, and autologous fascia, only mesh and autologous fascia showed no difference in tensile strength from baseline. Conversely, a significant loss of tensile strength and stiffness occurred in porcine and cadaveric materials within 12 weeks.16 The sterilization and storage process of allograft materials may impact mechanical properties. Freeze-dried cadaveric fascia was reported to be less stiff and had significantly lower maximum load to failure when compared to autologous rectus fascia, cadaveric dermis, and solvent dehydrated cadaveric fascia lata.17
DNA has been detected in freeze-dried or solvent-dehydrated cadaveric fascia lata and acellular dermis. The risk of human immunodeficiency virus transmission from frozen allografts has been estimated to be 1 in 8 million.18 Tissue rejection remains a concern with non-autologous tissues but has not specifically been reported to date.
The ideal biomaterials should meet several characteristics including being inert, sterile, non-carcinogenic, mechanically durable, liable to cause no/minimal inflammatory or immune reaction, inexpensive, convenient, easy to use, readily available, able to maintain implanted shape and configuration, able to withstand modification by body tissue (if synthetic), and able to resist enzymatic breakdown prior to established neovascularization and collagen in-growth (if biologic).19 Unfortunately, the perfect biomaterial which meets all these criteria, does not exist.
CLINICAL DATA ON GRAFT USE IN PELVIC RECONSTRUCTION
The limitations to assess efficacy and safety of synthetic or biological grafts in vaginal pelvic reconstructive surgery include variability in success and failure definitions, lack of functional outcomes reports, and lack of well-designed studies (especially randomized controlled trials and long-term follow-up periods). In the following review of clinical outcomes, results of cases with at least 1-year follow-up will be presented.
Anterior vaginal wall prolapse
The most common site of recurrent pelvic organ prolapse is the anterior compartment, with failure rates reported at 70%.20 Such high recurrence rates may be due to the use of attenuated tissue for plication or the failure to identify all existent defects. Richardson et al. showed that 70-80% of all cystoceles were due to lateral defects, in which lateral attachments of the vesicopelvic or anterior cardinal ligaments to the pelvic sidewall were disrupted.21 Combined central and lateral defects can occur in severe anterior vaginal prolapse. Frequent complications and technical difficulty with a vaginal approach to paravaginal repair have limited its widespread use in clinical practice. Even after paravaginal repair with anterior colporrhaphy, high recurrence of anterior vaginal prolapse has been noted.22
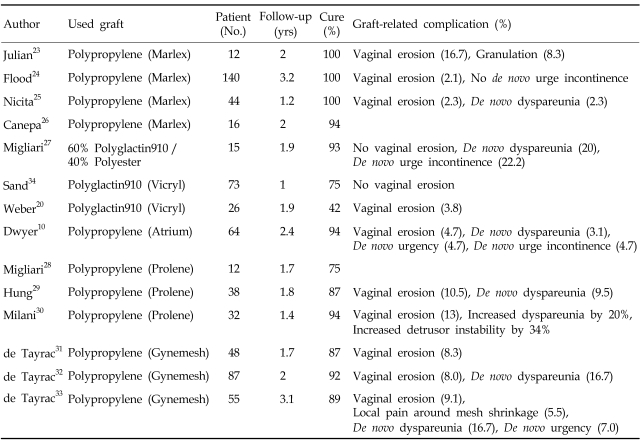
In 1996, Julian reported a prospective randomized study of synthetic, non-absorbable mesh (Marlex) in 24 women with recurrent anterior vaginal wall prolapse. After two years, 4 women in the control group and none in the mesh group had recurrent anterior vaginal wall prolapse. However, 3 (33%) patients had mesh-related complications; 2 with (16.7%) vaginal erosion, and 1 with (8.3%) granulation.23
Several other studies have shown that the use of non-absorbable synthetic mesh for anterior vaginal wall prolapse offers excellent anatomical cure rates.10,24-33 However, a high incidence of healing abnormalities has been noted, although most can be conservatively treated with local estrogen or simple excision of exposed mesh in the office setting. Functional adverse outcomes such as de novo urgency, urge incontinence, and de novo dyspareunia have also been frequently reported.
The use of absorbable synthetic mesh appears to lessen mesh-related complications, although it also decreases anatomical cure rates. In a prospective randomized controlled study, Sand et al. evaluated the efficacy of adding polyglactin 910 (Vicryl) to anterior colporrhaphy. Eighty women received mesh, and 80 did not. Preoperatively, 49 women had central cystocele to the hymenal ring, and 111 women had cystocele beyond the introitus. At 1-year follow-up, 30 (43%) of 70 subjects without mesh and 18 (25%) of 73 subjects with mesh had recurrent cystoceles to the mid-vaginal plane (p=0.02). Eight women without mesh and 2 women with mesh had recurrent cystoceles to the hymenal ring (p=0.04). No recurrent cystoceles beyond the hymenal ring occurred in either group.34
However, Weber et al. found no significant difference in recurrence of cystoceles in their prospective randomized study using the same mesh. One-hundred-fourteen women with anterior vaginal prolapse (mostly stage II and III, according to the pelvic organ prolapse quantification system) were assigned to undergo anterior repair by one of 3 techniques: standard, standard plus polyglactin 910 mesh, or ultralateral anterior colporrhaphy. One-hundred-nine patients underwent operation, 83 returned for follow-up. At median follow-up of 23.3 months, 10 (30%) of 33 patients assigned to the standard anterior colporrhaphy group experienced satisfactory (stage 0) or optimal (stage I) anatomical results, compared with 11 (42%) of 26 patients with standard plus mesh, and with 11 (46%) of 24 patients with ultralateral anterior colporrhaphy.20
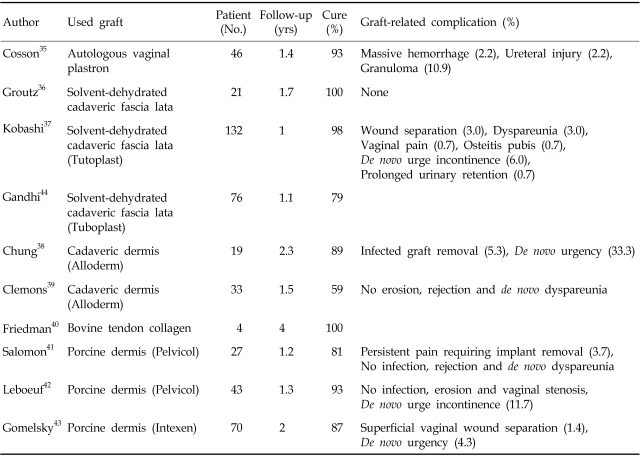
Several reports on biological grafts have shown excellent results with lower graft-related risks, except for the report of Clemons et al.35-43 However, the only well-designed study failed to demonstrate efficacy with allografts. In a prospective randomized trial, Gandhi et al. compared anterior colporrhaphy alone (n=78) and with augmented with fascia lata graft (n=76) for cystoceles of stage II or more. Sixteen (21%) women in the patch group and 23 (29%) in the control group experienced recurrent anterior vaginal wall prolapse, defined as stage II anterior vagina wall prolapse or worse (p=0.229).44 There are no randomized controlled or case-control studies on the use of xenografts.
Table 2 and 3 briefly summarize outcomes following use of grafts in anterior pelvic reconstruction.
Table 2.
Summary of Clinical Data on Use of Synthetic Mesh for Anterior Vaginal Wall Prolapse
Table 3.
Summary of Clinical Data on Use of Biological Grafts for Anterior Vaginal Wall Prolapse
Posterior vaginal wall prolapse
Traditional posterior colporrhaphy with or without levator ani plication has been reported to result in suboptimal (yet relatively acceptable compared to anterior colporrhaphy) long-term anatomical cure rates, and contribute to significant bowel and sexual dysfunction.45 Discrete site-specific repair, based on the concept of Richardson et al. is also reported to be suboptimal. In a retrospective case-control study, Abramov et al., noted a significantly higher anatomical recurrence rate of rectoceles and similar rates of dyspareunia and bowel symptoms following site-specific repair compared to traditional midline plication.46
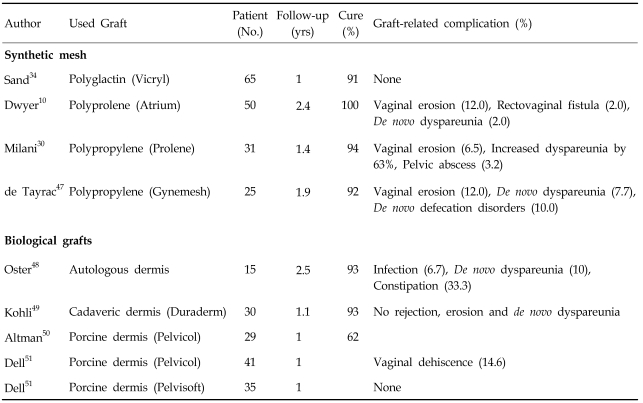
Reports on the use of grafts for posterior vaginal wall prolapse are more limited than those for anterior vaginal wall prolapse.10,30,34,47-51 Table 4 briefly summarizes outcomes following the use of grafts in posterior pelvic reconstruction.
Table 4.
Summary of Clinical Data on Use of Grafts for Posterior Vaginal Wall Prolapse
The use of synthetic mesh in posterior pelvic reconstruction has been associated with high incidence of vaginal erosion and functional adverse outcome as demonstrated in anterior pelvic reconstruction.10,30,34,47
The only well-designed study failed to evaluate the efficacy of mesh. In a prospective randomized controlled trial by Sand et al., 91 women had rectocele to the mid-vaginal plane, 31 to the hymenal ring, and 22 beyond the introitus. Sixty-five women received polyglactin 910 mesh, 67 did not. At 1 year, 4 (5.7%) subjects without mesh and 3 (4.1%) with mesh had recurrent rectoceles to mid-vaginal plane (p=0.66). An additional 3 women in each group had recurrent rectoceles beyond the hymenal ring (p=0.96).34 Based on current knowledge, the addition of synthetic grafts in posterior pelvic reconstruction does not appear to have any advantage over traditional procedures, unlike anterior pelvic reconstruction.
Only 4 studies on biological materials are available and there are no randomized controlled or case-control studies. Oster et al. described using submucosal dermis graft to reinforce the vaginal wall. Fifteen patients were followed up for 4 years. All patients were cured of symptoms, and anatomical results were less than perfect in only 1 case. However, significant graft-related complications were noted.48
Kohli et al. described their initial experience of rectocele repair using dermal allograft to augment site-specific fascial defect repair of rectovaginal repair. Forty-three women with advanced posterior vagina wall prolapse underwent dermal allograft augmentation. At an average of 12.9 months, 28 (93%) of 30 women were noted to have attained surgical cure, defined as a point Ap measurement of less than -0.5. No graft-related complications were noted.49
Altman et al. evaluated the functional and anatomical outcomes after transvaginal rectocele repair using porcine collagen mesh (Pelvicol) in 29 women. At 6- and 12-month follow-ups, 5 (17%) and 7 (24%) of 29 women had stage II or worse rectocele, respectively. Bowel symptoms were significantly improved at the 6-month follow-up, but were less pronounced at 12 months. They concluded there was substantial risk for recurrence with unsatisfactory anatomical and functional outcomes 1 year following surgery.50
Dell et al. reported their initial series of 35 patients treated with porcine dermal acellular collagen matrix biomesh (Pelvisoft), which alleviated problems with early postoperative vaginal mucosal dehiscence and delayed healing experienced with Pelvicol (14.6%). In their reports, anatomical cure rates were not presented, although overall improvement was noted. Average measurement of point Ap was 0.3 preoperatively and -2.3 postoperatively and point Bp was 1.2 preoperatively and -2.5 postoperatively.51
Vaginal vault prolapse
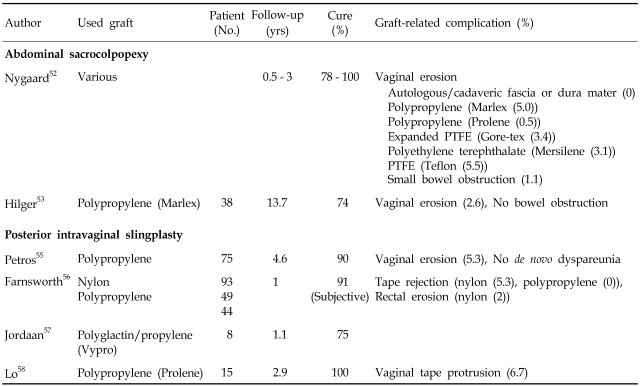
In abdominal sacrocolpopexy, the gold standard surgical procedure for vaginal vault prolapse, the use of grafts is proven to be effective and safe. In the literature review, Nygaard et al. presented satisfactory long-term anatomical cure rates with low incidence of mesh-related complications.52 They noted that vaginal mesh erosion was dramatically decreased with use of prolene mesh (0.5%) compared to other synthetic materials (3.1-5.5%). Hilger et al. presented a 74% success rate after abdominal sacrocolpopexy using polypropylene mesh (Marlex) at a mean follow-up of 13.7 years.53 The superior material for abdominal sacrocolpopexy has not been fully investigated. In a retrospective cohort study, Gregory et al., compared surgical outcomes of abdominal sacrocolpopexy with synthetic mesh to cadaveric fascia lata. Nineteen women who had abdominal sacrocolpopexy with synthetic mesh and 18 women who had abdominal sacrocolpopexy with freeze-dried, irradiated cadaveric fascia lata returned for blinded pelvic organ prolapse quantification examinations. The mean relative vaginal descent (delta) from perfect total vaginal length in the mesh group was 1.1cm, and in the fascia group was 2.8cm (p=0.02). The proportion of women with "optimal" surgical outcome, defined as a point C within 2cm from the total vaginal length, was 89% and 61% in the mesh and fascia group, respectively (p=0.06). They concluded that cadaveric fascia lata might not be an appropriate choice for abdominal sacrocolpopexy.54
Posterior intravaginal slingplasty, a new minimally invasive technique introduced by Petros et al. to eliminate the need for explo-laparotomy, and longer operation and admission times were studied.55 In 4 available studies, excellent anatomical cure rates were reported, however the studies had high rates of vaginal erosion, except for the report by Farnsworth et al., in spite of the use of prolene.56-8 Rectovaginal fistula and severe pain in the vagina or buttock/rectum, as well as dyspareunia have also been reported.59,60 For now, this procedure cannot be recommended until further data on efficacy and safety are available.
Table 5 briefly summarizes the outcomes following use of grafts in anterior pelvic reconstruction.
Table 5.
Summary of Clinical Data on Use of Grafts for Vaginal Vault Prolapse
PTFE, polytetrafluoroethylene.
DISCUSSION
The goal of pelvic reconstructive surgery is to correct anatomical and functional abnormalities, and to improve quality of life. Various graft materials have been used but do not offer satisfactory results.
The use of synthetic grafts has shown excellent anatomical cure rates but is associated with high graft-related complications, including healing abnormalities and adverse effects on bladder, bowel, and sexual functions. These complications may be lessened by absorbable mesh, although efficacy seems to decrease. In order to minimize the risk of vaginal erosions with synthetic meshes, several recommendations are proposed: preoperative estrogen therapy, strict aseptic conditions for mesh placement, intraoperative antibiotics, deep placement of mesh, optimal mesh adjustment to avoid folding, and limited colpectomy to avoid direct contact between vaginal scar and mesh during postoperative scar formation.49 There is insufficient evidence to favor biological grafts.
Based on current knowledge, the routine application of grafts in pelvic reconstruction is not recommended. It is preferred that graft utilization be individualized with close monitoring for complications.
Further studies are required to clarify specific situations in which grafts may be beneficial, and to achieve a better understanding of the associated risks, preferably in the setting of randomized controlled trials with proper follow-up periods. Surgeon education will be also required to prevent indiscrete use of grafts in vaginal pelvic reconstruction.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Shah DK, Paul EM, Rastinehad AR, Eisenberg ER, Badlani GH. Short-term outcome analysis of total pelvic reconstruction with mesh: the vaginal approach. J Urol. 2004;171:261–263. doi: 10.1097/01.ju.0000100141.38862.38. [DOI] [PubMed] [Google Scholar]
- 3.Amrute KV, Eisenberg ER, Rastinehad AR, Kushner L, Badlani GH. Analysis of outcomes of single polypropylene mesh in total pelvic floor reconstruction. Neurourol Urodyn. 2007;26:53–58. doi: 10.1002/nau.20362. [DOI] [PubMed] [Google Scholar]
- 4.Reisenauer C, Kirschniak A, Drews U, Wallwiener D. Anatomical conditions for pelvic floor reconstruction with polypropylene implant and its application for the treatment of vaginal prolapse. Eur J Obstet Gynecol Reprod Biol. 2007;131:214–225. doi: 10.1016/j.ejogrb.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Pulliam SJ, Ferzandi TR, Hota L, Rosenblatt PL. Use of synthetic mesh in pelvic reconstructive surgery: attitudes and practice patterns of urogynecologists. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(Suppl 3):S457–S458. doi: 10.1007/s00192-007-0360-6. [DOI] [PubMed] [Google Scholar]
- 6.Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997;1:15–21. [Google Scholar]
- 7.Bobyn JD, Wilson GJ, McGregor DC, Pilliar RM, Weatherly GC. Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants. J Biomed Mater Res. 1982;16:571–584. doi: 10.1002/jbm.820160505. [DOI] [PubMed] [Google Scholar]
- 8.Chvapil M, Holusa R, Kliment K, Stoll M. Some chemical and biological characteristics of a new collagen-polymer compound material. J Biomed Mater Res. 1969;3:315–332. doi: 10.1002/jbm.820030211. [DOI] [PubMed] [Google Scholar]
- 9.Chu CC, Welch L. Characterization of morphologic and mechanical properties of surgical mesh fabrics. J Biomed Mater Res. 1985;19:903–916. doi: 10.1002/jbm.820190803. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer PL, O'Reilly BA. Tranvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831–836. doi: 10.1111/j.1471-0528.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Kobashi KC, Dmochowski R, Mee SL, Mostwin J, Nitti VW, Zimmern PE, et al. Erosion of woven polyester pubovaginal sling. J Urol. 1999;162:2070–2072. doi: 10.1016/S0022-5347(05)68103-7. [DOI] [PubMed] [Google Scholar]
- 12.Rechberger T, Postawski K, Jakowicki JA, Gunja-Smith Z, Woessner JF., Jr Role of fascial collagen in stress urinary incontinence. Am J Obstet Gynecol. 1998;179:1511–1514. doi: 10.1016/s0002-9378(98)70017-1. [DOI] [PubMed] [Google Scholar]
- 13.Falconer C, Ekman G, Malmstrom A, Ulmsten U. Decreased collagen synthesis in stress-incontinent women. Obstet Gynecol. 1994;84:583–586. [PubMed] [Google Scholar]
- 14.McLennan MT, Bent AE. Fascia lata suburethral sling vs. Burch retropubic urethropexy. A comparison of morbidity. J Reprod Med. 1998;43:488–494. [PubMed] [Google Scholar]
- 15.Choe JM, Oqan K, Battino BS. Antimicrobial mesh versus vaginal wall sling: a comparative outcomes analysis. J Urol. 2000;163:1843–1844. [PubMed] [Google Scholar]
- 16.Dora CD, Dimarco DS, Zobitz ME, Elliott DS. Time dependent variations in biomechanical properties of cadaveric fascia, porcine dermis, porcine small intestine submucosa, polypropylene mesh and autologous fascia in the rabbit model: implications for sling surgery. J Urol. 2004;171:1970–1973. doi: 10.1097/01.ju.0000121377.61788.ad. [DOI] [PubMed] [Google Scholar]
- 17.Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;8:497–503. doi: 10.1002/(sici)1520-6777(1999)18:5<497::aid-nau12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Buck BE, Malinin TI. Human bone and tissue allografts. Preparation and safety. Clin Orthop Relat Res. 1994;303:8–17. [PubMed] [Google Scholar]
- 19.Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(Suppl 1):S51–S55. doi: 10.1007/s00192-006-0099-5. [DOI] [PubMed] [Google Scholar]
- 20.Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299–1306. doi: 10.1067/mob.2001.119081. [DOI] [PubMed] [Google Scholar]
- 21.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–362. [PubMed] [Google Scholar]
- 22.Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal wall segment: an analysis of support defects, operative morbidity, and anatomical outcome. Am J Obstet Gynecol. 1994;171:1429–1439. doi: 10.1016/0002-9378(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 23.Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472–1475. doi: 10.1016/s0002-9378(96)70092-3. [DOI] [PubMed] [Google Scholar]
- 24.Flood CG, Drutz HP, Waja L. Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:200–204. doi: 10.1007/BF01901604. [DOI] [PubMed] [Google Scholar]
- 25.Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741–745. doi: 10.1016/S0022-5347(01)62773-3. [DOI] [PubMed] [Google Scholar]
- 26.Canepa G, Ricciotti G, Introini C, Viqliercio G, Puppo P. Horseshoe-shaped marlex mesh for the treatment of pelvic floor prolapse. Eur Urol. 2001;39(Suppl 2):23–27. doi: 10.1159/000052554. [DOI] [PubMed] [Google Scholar]
- 27.Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255–1258. [PubMed] [Google Scholar]
- 28.Migliari R, De Angelis M, Madeddu G, Verdacchi T. Tension-free vaginal mesh repair for anterior vaginal wall prolapse. Eur Urol. 2000;38:151–155. doi: 10.1159/000020272. [DOI] [PubMed] [Google Scholar]
- 29.Hung ML, Liu FS, Shen PS, Chen GD, Lin LY, Ho ES. Factors that affect recurrence after anterior colporrhaphy procedure reinforced with four-corner anchored polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:399–406. doi: 10.1007/s00192-004-1185-1. [DOI] [PubMed] [Google Scholar]
- 30.Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107–111. doi: 10.1111/j.1471-0528.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 31.de Tayrac R, Gervaise A, Chauveaud-Lambling A, Fernandez H. Combined genital prolapse repair reinforced with a polypropylene mesh and tension-free vaginal tape in women with genital prolapse and stress urinary incontinence: a retrospective case-control study with short-term follow-up. Acta Obstet Gynecol Scand. 2004;83:950–954. doi: 10.1111/j.0001-6349.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- 32.de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75–80. [PubMed] [Google Scholar]
- 33.de Tayrac R, Deffieux X, Gervaise A, Chauveaud-Lambling A, Fernandez H. Long-term anatomical and functional assessment of trans-vaginal cystocele repair using a tension-free polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:483–488. doi: 10.1007/s00192-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 34.Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystocele and rectoceles. Am J Obstet Gynecol. 2001;184:1357–1362. doi: 10.1067/mob.2001.115118. [DOI] [PubMed] [Google Scholar]
- 35.Cosson M, Collinet P, Occelli B, Narducci F, Crepin G. The vaginal patch plastron for vaginal cure of cystocele. Preliminary results for 47 patients. Eur J Obstet Gynecol Reprod Biol. 2001;95:73–80. doi: 10.1016/s0301-2115(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 36.Groutz A, Chaikin DC, Theusen E, Blaivas JG. Use of cadaveric solvent-dehydrated fascia lata for cystocele repair--preliminary results. Urology. 2001;58:179–183. doi: 10.1016/s0090-4295(01)01177-3. [DOI] [PubMed] [Google Scholar]
- 37.Kobashi KC, Leach GE, Chon J, Govier FE. Continued multicenter followup of cadaveric prolapse repair with sling. J Urol. 2002;168:2063–2068. doi: 10.1016/S0022-5347(05)64296-6. [DOI] [PubMed] [Google Scholar]
- 38.Chung SY, Franks M, Smith CP, Lee JY, Lu SH, Chancellor M. Technique of combined pubovaginal sling and cystocele repair using a single piece of cadaveric dermal graft. Urology. 2002;59:538–541. doi: 10.1016/s0090-4295(01)01611-9. [DOI] [PubMed] [Google Scholar]
- 39.Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612–1619. doi: 10.1016/s0002-9378(03)00929-3. [DOI] [PubMed] [Google Scholar]
- 40.Friedman EA, Meltzer RM. Collagen mesh prosthesis for repair of endopelvic fascial defects. Am J Obstet Gynecol. 1970;106:430–433. doi: 10.1016/0002-9378(70)90372-8. [DOI] [PubMed] [Google Scholar]
- 41.Salomon LJ, Detchev R, Barranger E, Cortez A, Callard P, Darai E. Treatment of anterior vaginal wall prolapse with porcine skin collagen implant by the transobturator route: preliminary results. Eur Urol. 2004;45:219–225. doi: 10.1016/j.eururo.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Leboeuf L, Miles RA, Kim SS, Gousse AE. Grade 4 cystocele repair using four-defect repair and porcine xenograft acellular matrix (Pelvicol): outcome measures using SEAPI. Urology. 2004;64:282–286. doi: 10.1016/j.urology.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581–1584. doi: 10.1097/01.ju.0000115956.25769.0a. [DOI] [PubMed] [Google Scholar]
- 44.Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649–1654. doi: 10.1016/j.ajog.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 45.Kahn MA, Stanton SL. Posterior colporrhaphy: its effects on bowel and sexual function. Br J Obstet Gynaecol. 1997;104:82–86. doi: 10.1111/j.1471-0528.1997.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 46.Abramov Y, Gandhi S, Goldberg RP, Botros SM, Kwon C, Sand PK. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314–318. doi: 10.1097/01.AOG.0000151990.08019.30. [DOI] [PubMed] [Google Scholar]
- 47.de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:100–105. doi: 10.1007/s00192-005-1317-2. [DOI] [PubMed] [Google Scholar]
- 48.Oster S, Astrup A. A new vaginal operation for recurrent and large rectocele using dermis transplant. Acta Obstet Gynecol Scand. 1981;60:493–495. doi: 10.3109/00016348109155466. [DOI] [PubMed] [Google Scholar]
- 49.Kohli N, Miklos JR. Dermal graft-augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:146–149. doi: 10.1007/s00192-002-1013-4. [DOI] [PubMed] [Google Scholar]
- 50.Altman D, Zetterstrom J, Lopez A, Anzen B, Falconer C, Hjern F, et al. Functional and anatomic outcome after transvaginal rectocele repair using collagen mesh: a prospective study. Dis Colon Rectum. 2005;48:1233–1242. doi: 10.1007/s10350-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 51.Dell JR, O'Kelley KR. PelviSoft Biomesh augmentation of rectocele repair: the initial clinical experience in 35 patients. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:44–47. doi: 10.1007/s00192-004-1217-x. [DOI] [PubMed] [Google Scholar]
- 52.Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805–823. doi: 10.1097/01.AOG.0000139514.90897.07. [DOI] [PubMed] [Google Scholar]
- 53.Hilger WS, Poulson M, Norton PA. Long-term results of abdominal sacrocolpopexy. Am J Obstet Gynecol. 2003;189:1606–1611. doi: 10.1016/j.ajog.2003.10.689. [DOI] [PubMed] [Google Scholar]
- 54.Gregory WT, Otto LN, Berqstrom JO, Clark AL. Surgical outcome of abdominal sacrocolpopexy with synthetic mesh versus abdominal sacrocolpopexy with cadaveric fascia lata. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:369–374. doi: 10.1007/s00192-004-1257-2. [DOI] [PubMed] [Google Scholar]
- 55.Petros PE. Vault prolapse II: Restoration of dynamic vaginal supports by infracoccygeal sacropexy, an axial day-case vaginal procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:296–303. doi: 10.1007/pl00004039. [DOI] [PubMed] [Google Scholar]
- 56.Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:4–8. doi: 10.1007/s001920200001. [DOI] [PubMed] [Google Scholar]
- 57.Jordaan DJ, Prollius A, Cronje HS, Nel M. Posterior intravaginal slingplasty for vaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:326–329. doi: 10.1007/s00192-005-0007-4. [DOI] [PubMed] [Google Scholar]
- 58.Lo TS, Horng SG, Huang HJ, Lee SJ, Liang CC. Repair of recurrent vaginal vault prolapse using sacrospinous ligament fixation with mesh interposition and reinforcement. Acta Obstet Gynecol Scand. 2005;84:992–995. doi: 10.1111/j.0001-6349.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 59.Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:89–92. doi: 10.1007/s00192-005-1354-x. [DOI] [PubMed] [Google Scholar]
- 60.Baessler K, Hewson AD, Tunn R, Schuessler B, Maher CF. Severe mesh complications following intravaginal slingplasty. Obstet Gynecol. 2005;106:713–716. doi: 10.1097/01.AOG.0000177970.52037.0a. [DOI] [PubMed] [Google Scholar]