Figure 5.
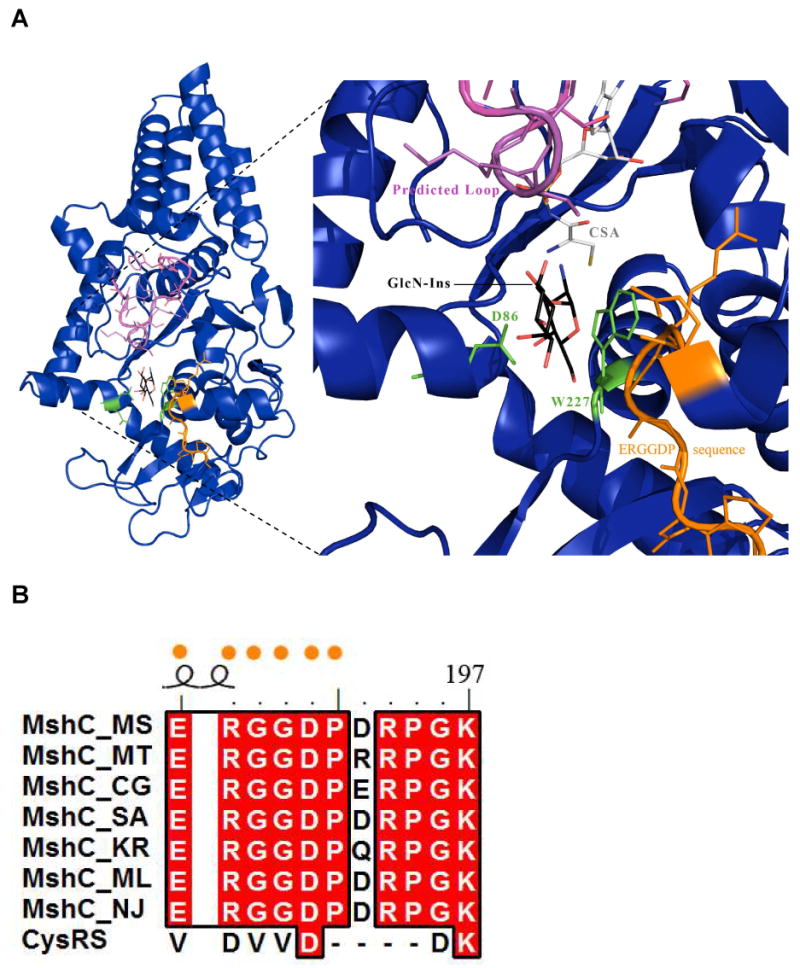
Active site docking of GlcN-Ins as well as modeling of the proteolized MshC loop segment based on the CysRS-tRNAcys (1U0B) structure. A) The modeled conformation of the proteolized loop (pink) on MshC (deep blue). The loop is positioned directly over the GlcN-Ins molecule (carbons colored black). Conserved residues D86 and W227 are shown in green, while the MshC ortholog identifying sequence is displayed in orange. These features likely bind and coordinate the GlcN-Ins molecule in the active site for catalysis. B) Sequence alignment of MshC orthologs and CysRS. The ERGGDP sequence (orange dots) is highly conserved amongst MshC orthologs but absent in the CysRS homologues.
