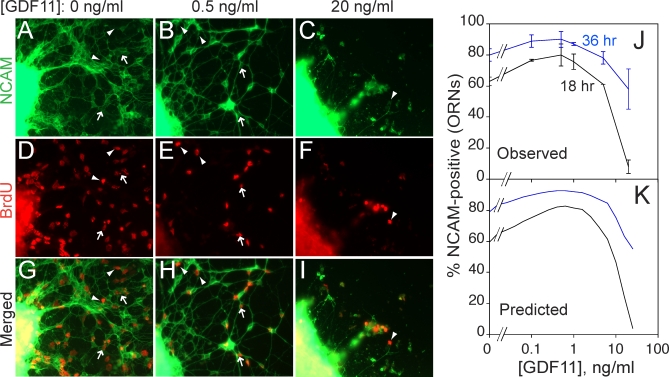
Figure 4. Experimental Demonstration That GDF11 Regulates p 1 and v 1 .
OE explants were cultured in various doses of GDF11. At 12 h, BrdU was added for 2 h and then washed out. Explants were fixed at various times after BrdU addition and immunostained for BrdU and NCAM expression.
(A–I) Cultures grown in GDF11 concentrations of 0 (A, D, and G), 0.5 (B, E, and H), and 10 (C, F, and I) ng/ml, fixed 18 h after BrdU addition (previous studies have shown that 18 h is sufficient time for INP progeny that become ORNs to express NCAM [39]). NCAM immunofluorescence (green) is shown in (A–C); BrdU immunofluorescence (red) in (D–F); merged images in (G–I). Arrowheads point to examples of BrdU+/NCAM− cells; arrows point to examples of BrdU+/NCAM+ cells.
(J) Percentage of BrdU+ cells migrating out of OE explants that had differentiated (acquired NCAM immunoreactivity) by 18 h (black line) or 36 h (blue line), as a function of GDF11 dose. Low doses of GDF11 increase the proportion of INP progeny that differentiate (i.e., p 1 decreases). At high dose, the effect reverses, with the NCAM+ fraction falling to near zero at 18 h, but recovering at 36 h. These data are consistent with a slowing of the cell cycle (v 1) such that 18 h is not long enough to produce NCAM+ offspring (but 36 h is). This interpretation is consistent with a previous demonstration that high doses of GDF11 reversibly arrest the INP cell cycle [34].
(K) Simulation of the experiment in (J) by a model in which GDF11 affects both p 1 and v 1. Parameters used in the model are consistent with measured proportions of ORNs, INPs, and Mash1 +/Sox2 + cells, as well as experimental data on the effects of GDF11 on BrdU pulse-labeling by INPs [34,39,40].