Abstract
Hippocampal inputs to the nucleus accumbens (noradrenaline, NA) have been proposed to implement a gating mechanism by driving NA medium spiny neurons (MSNs) to depolarized up states that facilitate action potential firing in response to brief activation of the prefrontal cortex (PFC). Brief PFC stimulation alone, on the other hand, could not drive NA up states. As these studies were conducted using single-pulse PFC stimulation, it remains possible that PFC activation with naturalistic, bursty patterns can also drive up states in NA MSN. Here, we assessed NA responses to PFC stimulation with a pattern similar to what is typically observed in awake animals during PFC-relevant behaviors. In vivo intracellular recordings from NA MSN revealed that brief 20–50 Hz PFC stimulus trains evoked depolarizations that were similar to spontaneous up states in NA MSN and were sustained beyond stimulus offset. Similar train stimulation of cortico-accumbens afferents in a parasagittal slice preparation evoked large amplitude depolarizations in NA MSN that were sustained during stimulation but decayed rapidly following stimulation offset, suggesting that activation of cortical afferents can drive MSN depolarizations but other mechanisms may contribute to sustaining up states. These data suggest that NA MSNs integrate temporal features of PFC activation and that the NA gating model can be reformulated to include a PFC-driven gating mechanism during periods of high PFC firing, such as during cognitively demanding tasks.
Keywords: ventral striatum, electrophysiology, intracellular, rat, train stimulation
INTRODUCTION
The noradrenaline (NA) lies at the confluence of massive cortical and subcortical inputs (Bolam et al., 2000; Groenewegen et al., 1990; Haber et al., 2000; McFarland and Haber, 2000). Individual NA medium spiny neurons (MSNs) receive convergent glutamatergic inputs from the prefrontal cortex (PFC), hippocampus, and amygdala (French and Totterdell, 2002, 2003; O’Donnell and Grace, 1995), as well as GABAergic and modulatory inputs from multiple sources (Meredith, 1999). Subthreshold membrane fluctuations are essential for integrating information carried by such inputs, as MSN have highly negative resting membrane potentials (down state) and must be driven to depolarized potentials (up state) in order for action potentials to be generated (Goto and O’Donnell, 2001; O’Donnell and Grace, 1995; Wilson, 1995). Although hippocampal inputs can gate cortical throughput by driving accumbens MSNs into sustained up states (O’Donnell and Grace, 1995), it is also possible that strong or sustained PFC activation may provide sufficient glutamatergic drive to evoke up states and gate inputs to the NA.
The in vivo response of MSNs to single-pulse electrical cortical stimulation has been well characterized in both dorsal (Wickens and Wilson, 1998; Wilson et al., 1983) and ventral striatum (Brady and O’Donnell, 2004; Goto and O’Donnell, 2002b; O’Donnell and Grace, 1995). However, PFC neurons often fire bursts of action potentials during PFC-relevant behaviors (Chafee and Goldman-Rakic, 1998; Chang et al., 2002; Peters et al., 2005; Tremblay and Schultz, 2000; Watanabe and Niki, 1985). Stimulation of cortical afferents with multipulse trains evoked persistent depolarizations in MSNs in corticostriatal and NA slice preparations (Lape and Dani, 2004; Vergara et al., 2003), suggesting that bursty PFC activity could drive NA up states. The impact of this bursting activity on MSN in vivo is not known. Here, we tested whether PFC stimulation with trains of pulses that mimic naturalistic PFC firing patterns can evoke persistent depolarizations in NA MSNs from anesthetized rats.
MATERIALS AND METHODS
In vivo recordings
Intracellular in vivo recordings were conducted in 13 male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 270–430 g. Rats were allowed ad libitum access to food and water and maintained on a 12 h light/dark cycle. These experiments were conducted in accordance with the United States Public Health Service Guide for the Use and Care of Animals, and all procedures were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Rats were initially anesthetized with chloral hydrate (400 mg/kg, i.p.), and subsequent anesthesia was maintained via constant infusion of chloral hydrate (20–30 mg/h, i.p.) using a syringe pump (Bioanalytical Systems, West Lafayette, IN). Rats were placed in a stereotaxic apparatus (David Kopf, Tujunga, CA), and an array of electrodes was implanted in the right prelimbic cortex (centroid of array tips with respect to bregma: 3.2 mm rostral and 0.6 mm lateral; depth: 4.3 mm ventral from skull; 30° angle toward midline). Electrode arrays consisted of 3–6 pairs of monopolar electrodes glued together with an approximate tip separation of 200 μm. Electrode pairs were made of commercially available tungsten electrodes (WPI, Sarasota, FL) or constructed with 115 μm-diameter Teflon-coated tungsten wire (AM Systems, Carlsborg, WA). Electrode pairs were mounted such that they were separated by approximately 500–750 μm in the sagittal plane. A custom switchbox was used to select electrode pairs for stimulation without mechanical disturbance to the array, and it was connected to an Isoflex stimulus isolation unit (AMPI, Jerusalem, Israel) driven by a Master-8 stimulator (AMPI). Electrical stimulation consisted of a train of 1–10 pulses, delivered at either low (20 Hz) or high (50 or 62 Hz) frequency. Pulses were 150 μs in duration, and had a maximal current of 0.2–1.0 mA. The responses of MSNs to stimulation through each of the electrode pairs in the array were assessed at the beginning of each recording session. The pair through which up states could be evoked with the least stimulation current was subsequently used throughout the session. Stimulation current was chosen at the beginning of each recording session to be slightly greater than the minimal current needed to evoke up states for each MSN, and was maintained at this level throughout the session.
Intracellular recording electrodes were pulled from 1 mm (o.d.) borosilicate glass tubing (WPI) to a resistance of 40–100 MΩ with a P-97 Flaming-Brown micro-electrode puller (Sutter Instruments, Novato, CA). Recording electrodes were filled with 2% Neurobiotin (Vector Laboratories, Burlingame, CA) in 2 M potassium acetate and lowered into the right NA with a hydraulic microdrive (Trent Wells, Coulterville, CA) within the following range of coordinates: 1.3–1.7 mm rostral to bregma, 1.2–1.4 mm lateral to the midline, and 5.5–7.5 mm ventral from the cortical surface (Fig. 1A). Intracellular signals from the recording electrode were amplified (Neurodata IR-283; Cygnus Tech, Delaware Water Gap, PA), low pass filtered at 2 kHz (FLA-01, Cygnus Tech.), digitized (DigiData 1322A, Axon Instruments, Union City, CA), sampled at 10 kHz using Axoscope (Axon Instruments) and stored on a PC. Once a neuron was impaled and the membrane potential stabilized, 5 min of background activity were recorded prior to any stimulation. For a neuron to be included in the present data set, its resting membrane potential (down state) had to be less than −60 mV, its action potential amplitude greater than 40 mV measured from threshold, and input resistance greater than 35 MΩ.
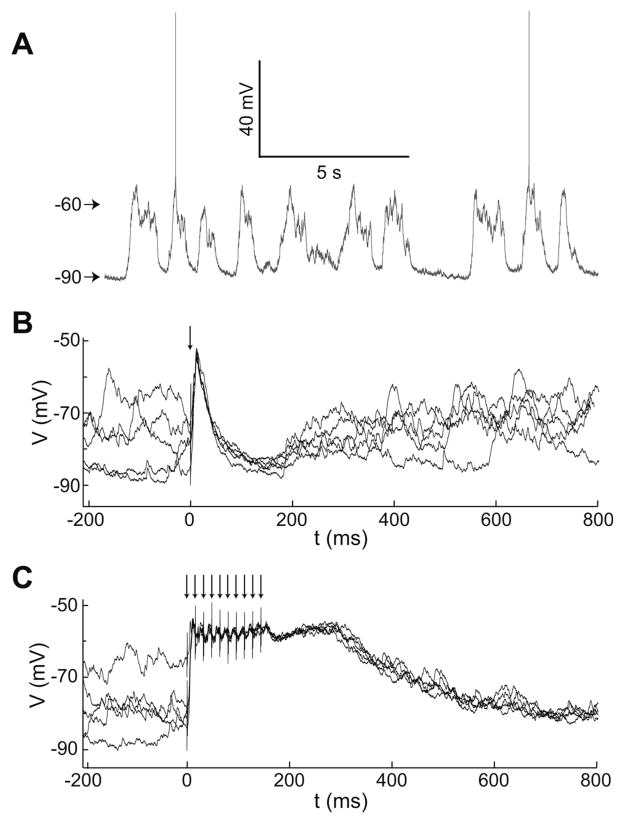
Fig. 1.
Intracellular recordings of MSNs in vivo. (A) Membrane potential trace from a representative MSN recorded in vivo showing spontaneous fluctuations between hyperpolarized and depolarized potentials. (B) Overlay of traces showing the response of a representative MSN to multiple trials of single-pulse electrical stimulation of PFC. (C) Overlay of traces showing the response of the same MSN as in (B) to multiple trials of PFC stimulation with a 10-pulse train. Arrows indicate times of stimulation pulses in this and in subsequent figures.
Following electrophysiological recordings Neurobiotin was injected into cells by passing positive current (0.5–1.0 nA, 200 ms pulses at 2 Hz) for 5–15 min through the recording electrode. At the completion of the experiments, rats were euthanized with an overdose of pentobarbital (100 mg/kg) and transcardially perfused with cold saline followed by 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde for at least 24 h before being transferred to a solution of 30% sucrose in 0.1 M phosphate buffer for cryoprotection. Sections were cut (30–40 μm) using a freezing microtome and placed in phosphate buffer. Sections through the PFC were mounted on gelatin-coated slides and Nissl-stained to verify placement of stimulating electrodes. Sections through the NA were processed for visualization of Neurobiotin-filled cells.
In vitro recordings
Parasagittal brain slices containing the NA were obtained from developmentally mature (postnatal day 52–66; n = 6) male Sprague-Dawley rats. Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.1 CaCl2, 3 MgCl2, pH 7.40; osmolarity 285–295 mOsm. Brains were removed and parasagittal slices (350 μm thick, 1.2–2.0 mm lateral from midline) containing PFC fibers projecting to the NA were cut on a Vibratome in ice-cold ACSF and immediately transferred and incubated in warm (35°C) ACSF solution constantly oxygenated with 95% O2 to 5% CO2 for at least 70 min before recording. Following incubation, slices were transferred to a recording chamber in which ACSF was perfused (2 ml/min.). For the recording ACSF, CaCl2 was increased to 2 mM and MgCl2 was decreased to 1 mM. All experiments were conducted at 33–35°C. Whole-cell recordings were performed from MSN in the NA core, identified under visual guidance using infrared differential interference contrast video microscopy. Electrical stimulation (50 Hz, 0.2–0.9 mA) was applied through an electrode consisting of a twisted pair of Teflon-coated tungsten wire. The stimulating electrode was placed near the forceps minor and whole-cell recordings were made 700–1000 μm caudally along intact corticostriatal fibers originating near the electrode site. Patch pipettes (10–16 MΩ) were filled with (in mM): 115 K-gluconate, 10 HEPES, 2MgCl2, 20 KCl, 2 MgATP, 2 Na2-ATP, 0.3 GTP, pH 7.3; 285–295 mOsm. CNQX was dissolved in ACSF and applied in the recording solution in known concentrations. Both control and drug-containing ACSF were oxygenated continuously throughout the experiments. The liquid junction potential (calculated to be +10.9 mV) was corrected post hoc.
Data analysis was performed with Axon Instruments ClampFit, Microsoft Excel, and Mathworks Matlab software. Statistical significance of data was evaluated by Student’s t-test unless otherwise stated.
RESULTS
In vivo intracellular recordings were conducted in the ventromedial striatum of mature rats. Recorded neurons were located primarily in the NA core, and a few were located dorsal to the classic NA boundary, but within the region receiving afferents from the medial PFC (Voorn et al., 2004). Inclusion of neurons in this study (n = 16) was determined by cell health, responsiveness to cortical stimulation, and posthoc verification of recording and stimulation electrode placement. Nine of these neurons were processed for Neurobiotin labeling and were morphologically identified as MSN. Input resistance was 52.8 ± 18.0 MΩ (mean ± SD) and action potential amplitude was 58.4 ± 8.7 mV measured from threshold. Most neurons (11/16) exhibited spontaneous transitions between down and up states (Fig. 1A). Down states were −83.1 ± 3.8 mV and up states were observed at −66.2 ± 2.1 mV with a duration of 391 ± 132 ms and at a frequency of 0.68 ± 0.2 Hz. Spontaneous firing was detected in 7 of 16 neurons at 0.85 ± 1.12 Hz. These properties are similar to what was previously reported in the NA (Brady and O’Donnell, 2004; O’Donnell and Grace, 1995).
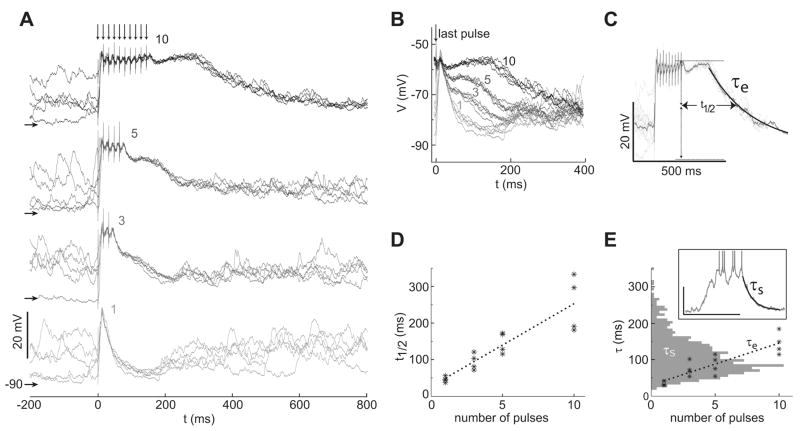
Different patterns of stimulation were used to characterize temporal aspects of NA MSN responses to PFC activation. Arrays of stimulating electrodes were placed within the prelimbic region of the medial PFC. One pair of electrodes in the array was selected for delivering electrical stimulation. Single electrical pulses evoked large amplitude depolarizations followed by hyperpolarizations in NA MSNs (n = 6 of 6; Fig. 1B). This response is identical to what was previously reported in NA and dorsal striatal MSNs (O’Donnell and Grace, 1995; Wickens and Wilson, 1998; Wilson et al., 1983). When the PFC was stimulated with trains of pulses, a qualitatively different pattern of responses emerged. High frequency trains (10 pulses at 50 or 62 Hz) were highly effective for driving sustained depolarizations from baseline (−84.9 ± 4.9 mV) to the up state (−58.1 ± 7.0 mV; Fig. 1C). Similar results were obtained with 3, 5, or 10 pulse trains (Fig. 2A). The trajectory of the evoked response was reliable over multiple trials irrespective of the previous up/down state of the neuron, indicating that the stimulation was able to temporarily override the ongoing cortical activity despite the phase of the global cortical state, as recently shown in the dorsal striatum for single-pulse cortical stimulation (Kasanetz et al., 2006). However, the membrane potential trajectory following the last stimulation pulse depended on the number of pulses in the train (Fig. 2B). To quantify the duration of the depolarization, we (i) computed the decay time to half amplitude, and (ii) fit a single exponential function to the decaying portion of the transition (Fig. 2C). Both measures showed a significant linear correlation with the number of pulses in the stimulus train (r2 = 0.81, and r2 = 0.78, respectively; n = 4; P < 0.01 for both; Figs. 2D and 2E), indicating that more pulses in the train lead to longer membrane depolarizations following stimulus offset. Furthermore, decay times of evoked responses and spontaneous transitions from up to down states fell within the same range (20–300 ms; Fig. 2E), suggesting that these events may terminate by a common mechanism. These data indicate that high-frequency train stimulation of PFC can drive sustained depolarizations in NA MSN that are similar to spontaneous up states and that the duration of the NA depolarizations depends on the length of the persistent PFC activity.
Fig. 2.
Dependence of sustained depolarizations on the number of stimulation pulses. (A) Overlay of traces showing the response of a representative MSN to stimulation trains with 1, 3, 5, and 10 pulses (from bottom to top and light to dark traces). Traces are aligned in time to the onset of the first stimulation pulse, and responses to each train length are aligned in the voltage axis (horizontal arrows indicate −90 mV for each response group). (B) The same traces in A aligned to the last stimulation pulse and to a common voltage axis, showing that the trajectory following stimulation offset depends on the number preceding electrical pulses. (C) Illustration of the response features characterized by the time to half decay (t1/2) and decay time constant of the evoked response (τe), superimposed on overlays of the evoked voltage traces showing the response of a MSN to train stimulation. (D) Plot of the time to half decay and the number of pulses for multiple cells revealing a significant linear correlation (n = 4; r2 = 0.81; P < 0.01). (E) Decay time constant (τe) was also correlated with the number of pulses across cells (n = 4; r2 = 0.78; P < 0.01). The graph shows the distribution of decay time constants for the range of pulses tested (asterisks), overlaid with the distribution of decay time constants (horizontal histogram) measured during spontaneous up-down transitions (n = 11). Inset shows an example of τs calculated from an up-down transition.
We also explored whether the frequency of PFC stimulus trains affected the duration of evoked depolarizations. Stimulation at lower frequencies (20 Hz) revealed depolarizing postsynaptic potentials following each stimulation pulse (Fig. 3A), but led to shorter sustained depolarizations following the 10th pulse (t1/2 = 105.3 ± 63.4 ms; τe = 84.4 ± 49.9 ms), than did high frequency (50 Hz) stimulation in the same neurons (t1/2 = 183.9 ± 80.4 ms; τe = 149.1 ± 34.2 ms; n = 4; P < 0.01 for both; Figs. 3B–3D). Thus, NA MSN persistent depolarizations were more readily evoked with higher-frequency cortical stimulation.
Fig. 3.
Dependence of sustained depolarizations on stimulation frequency. (A) Overlay of traces from a representative NA neuron showing the subthreshold response to lower frequency stimulation (20 Hz), revealing postsynaptic potentials in response to every pulse in the train. This indicates effective cortico-accumbens transmission throughout the train. (B) Overlay of traces from a representative MSN showing the membrane potential trajectory following stimulation offset for 10-pulse trains at higher frequency (62 Hz, dark traces) and lower frequency (20 Hz, gray trace). (C) Time to half decay is reduced for lower frequency stimulation (n = 4; P < 0.01). (D) Decay time constants are also reduced for lower frequency stimulation (n = 4; P < 0.01).
MSNs are endowed with a number of voltage-gated membrane conductances that activate at depolarized potentials and could influence sustained depolarizations following stimulation offset. To explore this possibility, we repeated high-frequency stimulation in some MSN while depolarizing artificially with current injection through the recording electrode. Such depolarization significantly increased the half decay time PFC-evoked responses from 270.4 ± 63.9 ms to 398.8 ± 83.8 ms (n = 4; P = 0.038), and the decay time constant showed a nonsignificant trend toward higher values from 149.7 ± 25.6 ms to 308.5 ± 100.5 ms (n = 4; P = 0.066). This shift toward longer evoked responses under artificial depolarization indicates that intrinsic membrane properties may contribute to sustaining evoked depolarizations.
Although glutamate release from cortico-accumbens projections provides a direct mechanism by which cortical stimulation could evoke up states, the stimulation may evoke excitation indirectly by driving activity in other structures that provide glutamatergic inputs to the NA such as the hippocampus, amygdala, thalamus, or ventral tegmental area. To test whether PFC afferents alone can provide sufficient excitatory input to drive sustained up states, we performed whole-cell recordings from 17 MSNs (Fig. 4A) in parasagittal slices that preserved cortico-accumbens afferents but excluded nonstriatal structures (O’Donnell and Grace, 1994). These neurons had a resting membrane potential of −86.6 ± 2.2 mV and input resistance of 83 ± 26 MΩ. Electrical stimulation of cortico-accumbens fibers with a train of pulses similar to that used in the in vivo recordings (10 pulses at 50 Hz; 0.2–1.0 mA) evoked robust depolarizing responses (14.3 ± 4.6 mV, Fig. 4B) in MSNs located 0.5–1 mm away along intact fibers originating near the stimulation site. Bath application of the AMPA antagonist CNQX (10 μM) greatly attenuated evoked responses in all cells tested (85% ± 10% reduction; P < 0.001; n = 7; Fig. 4B), confirming them to be synaptically driven. Evoked depolarizations were sustained throughout stimulation, but began a rapid decay to resting membrane potential soon after stimulation offset (t1/2 = 96 ± 28 ms; n = 17; Fig. 4C). Depolarization of MSNs (ΔV from baseline = 14.0 ± 4.4 mV) by current injection through the recording electrode revealed a significantly slower repolarization (t1/2 = 181 ± 90 ms; P < 0.001 by paired t-test; n = 17; Fig. 4D), consistent with the effect of current injection in vivo. Thus, stimulation of cortical afferents in vitro with the pattern and intensity of electrical pulses used for evoking responses in vivo is sufficient to drive large amplitude depolarizations that bring NA MSN close to the up state, but evoked responses in slices were briefer than those evoked in vivo.
Fig. 4.
Stimulation of corticostriatal fibers evokes up states in a parasagittal slice preparation. NA spiny neurons were recorded in a whole-cell patch clamp configuration of 0.5–1.0 mm away from the stimulation site along intact corticostriatal fibers. (A) Overlay of voltage responses (top left) to intracellular current pulses (bottom left). Right: I/V plot revealing the characteristic inward rectification in the hyperpolarizing direction. (B) Overlay of average voltage traces recorded in current-clamp mode from a representative NA spiny neuron shows that train stimulation of corticostriatal afferents (arrows) can drive large depolarizations from the hyperpolarized resting down state (dark trace) and that these are greatly attenuated by bath application of the AMPA antagonist CNQX (10 μM; gray trace). (C) Overlay of average voltage traces from a different neuron, showing that depolarization by constant current injection through the recording electrode (upper gray trace) induces a slower repolarization of evoked responses following stimulation offset than responses without such depolarization (lower dark trace). (D) Bar plot showing means and 95% confidence intervals of time to half decay for NA MSN evoked responses in vivo and in the slice preparation with (shaded bars) and without depolarization by current injection through the recording electrode.
DISCUSSION
PFC stimulation with trains of electrical pulses that mimic bursty PFC activity in behaving animals evoked prolonged depolarizations that were similar to spontaneous up states in NA MSNs. These evoked depolarizations were sustained beyond stimulation offset when evoked with high frequency trains in vivo, and the duration of depolarization following stimulation offset depended on the number of pulses in the train. Stimulation of cortico-accumbens afferents in a parasagittal slice preparation also evoked large amplitude depolarizations in NA MSN. These data reveal that strong PFC activation is able to drive depolarizations similar to up states in NA MSNs and indicate that NA MSNs integrate temporal features of cortical activation.
This integration of cortical information in the NA may be influenced by intrinsic membrane properties of MSNs. Recordings in corticostriatal and NA slice preparations have shown that electrical train stimulation of cortical afferents can induce sustained depolarizations in MSNs that are enhanced by L-type Ca2+ and NMDA conductances (Lape and Dani, 2004; Vergara et al., 2003), which are activated at depolarized potentials (Bargas et al., 1994; Calabresi et al., 1992). Here, artificial depolarization of MSNs with current injection through the recording electrode yielded longer persistent depolarizations in vivo and in vitro, suggesting that voltage-gated currents may contribute to sustaining the evoked responses.
Network factors could also contribute to the sustained evoked depolarizations. Slice recordings revealed briefer PFC-evoked depolarizations in NA neurons than in vivo recordings. This difference could be due to reduced afferent activity in the slice preparation that may alter tonic or evoked levels of neuro-transmitters or modulators. Reverse dialysis revealed that burst PFC stimulation in vivo can increase levels of many transmitters and modulators in the NA, such as dopamine (DA) (You et al., 1998), acetylcholine (Consolo et al., 1996), and cholecystokinin (You et al., 1998). It is also possible that PFC stimulation orthodromically or antidromically activates glutamatergic afferents to the NA, such as those arising in the hippocampus, thalamus, or amygdala. However, several lines of evidence suggest that only cortical afferents are selectively activated by the stimulation in the in vitro preparation. Evoked responses were only observed when the stimulating electrode and patched neuron were along the same visually identified cortico-accumbens fiber tract, indicating that it is activation of these tracts that mediates the evoked responses rather than nonselective activation of terminals nearby the patched neuron. In addition, CNQX was highly effective in attenuating evoked responses, suggesting that direct current innervation is not large in the vicinity of the recorded neuron, and thus is not expected to activate nearby afferents that do not pass near the recording electrode. Furthermore, cortical projections are spatially segregated from hippocampal and other inputs (Charara and Grace, 2003), indicating that cortical afferents are selectively activated by the stimulation. Thus, the findings that cortical afferent stimulation evokes large amplitude depolarizations in the slice preparation and the attenuation of these responses by CNQX application, suggest that glutamate release by cortical afferents can drive NA MSNs into the up state during periods of strong cortical activity.
Our finding that bursts of PFC stimulation are sufficient to evoke sustained depolarizations in MSNs indicates that the gating model in the NA can be reformulated to also include a PFC-driven gating mechanism during periods of high PFC firing. Input from PFC and hippocampus may not gate NA activity equivalently, as hippocampal activation enhances subsequent PFC-evoked post synaptic potentials, while PFC activation fails to similarly enhance hippocampal-evoked responses (Goto and O’Donnell, 2002b). Furthermore, hippocampal lesions eliminate spontaneous up states in NA MSNs (Goto and O’Donnell, 2002a; O’Donnell and Grace, 1995), indicating that spontaneous PFC activity is not sufficient to drive up states. However, conditions that strongly engage PFC, such as tasks involving set shifting and working memory (Chafee and Goldman-Rakic, 1998; Chang et al., 2002; Rogers et al., 2000), may provide sufficient PFC output to gate NA activity during a brief temporal window.
The ability of either hippocampus or PFC to gate NA activity could allow for dynamic gating in the NA based on the functional processing in either input structure. Indeed, lesion studies have shown that disruption cortico-accumbens circuits selectively disrupts working memory and set-shifting, whereas disruption of hippocampal-accumbens circuits selectively disrupts behaviors that rely on long-term memory and self-guided foraging (Block et al., 2007; Floresco et al., 1997; Goto and Grace, 2005; Seamans and Phillips, 1994). Sustained MSN depolarizations may be computationally important by helping entrain MSNs to oscillating input (Wolf et al., 2005), enhance the detection of signals from noisy backgrounds (Gruber et al., 2003), and may provide a temporal window for integration of inputs from multiple afferent structures. Thus, dynamic gating in the NA may be important for the cognitive flexibility needed for guiding behavior in a variety of environments that may require either self-directed actions based on past experience or active processing of complex sensory information.
Acknowledgments
Contract grant sponsor: USPHS grant; Contract grant number: MH60131; Contract grant sponsor: Tourette Syndrome Association Award.
References
- Bargas J, Howe A, Eberwine J, Surmeier JD. Cellular and molecular characterization of CA2+ currents in acutely isolated. Adult rat neostraial neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organization of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and pariental area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: Ensemble recording in freely moving rats. Exp Brain Res. 2002;142:67–80. doi: 10.1007/s00221-001-0918-3. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28:1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Consolo S, Baldi G, Giorgi S, Nannini L. The cerebral cortex and parafascicular thalamic nucleus facilitate in vivo acetylcholine release in the rat striatum through distinct glutamate receptor subtypes. Eur J Neurosci. 1996;8:2702–2710. doi: 10.1111/j.1460-9568.1996.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neurosci. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Synchronous activity in the hippocampus and nucleus accumbens in vivo. J Neurosci. 2001;21:RC131. doi: 10.1523/JNEUROSCI.21-04-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002a;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. Proc Natl Acad Sci USA. 2002b;99:13189–13193. doi: 10.1073/pnas.202303199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: Evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Solla SA, Houk JC. Dopamine induced bistability enhances signal processing in spiny neurons. In: Becker S, Saul L, Obermayer K, editors. Advances in neural information processing systems. Vol. 15. Cambridge, MA: MIT Press; 2003. pp. 165–172. [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, O’Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. J Physiol. 2006;577(Pt 1):97–113. doi: 10.1113/jphysiol.2006.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Dani JA. Complex response to afferent excitatory bursts by nucleus accumbens medium spiny projection neurons. J Neurophysiol. 2004;92:1276–1284. doi: 10.1152/jn.00066.2004. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: Hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–468. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J Neurophysiol. 2000;83:1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553(Pt 1):169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Niki H. Hippocampal unit activity and delayed response in the monkey. Brain Res. 1985;325:241–254. doi: 10.1016/0006-8993(85)90320-8. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol. 1998;79:2358–2364. doi: 10.1152/jn.1998.79.5.2358. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The contribution of cortical neurons to the firing pattern of striatal spiny neurons. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge: MIT Press; 1995. [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res. 1983;51:227–235. doi: 10.1007/BF00237198. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo micro-dialysis study in freely moving rats. J Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]