Fig. 3.
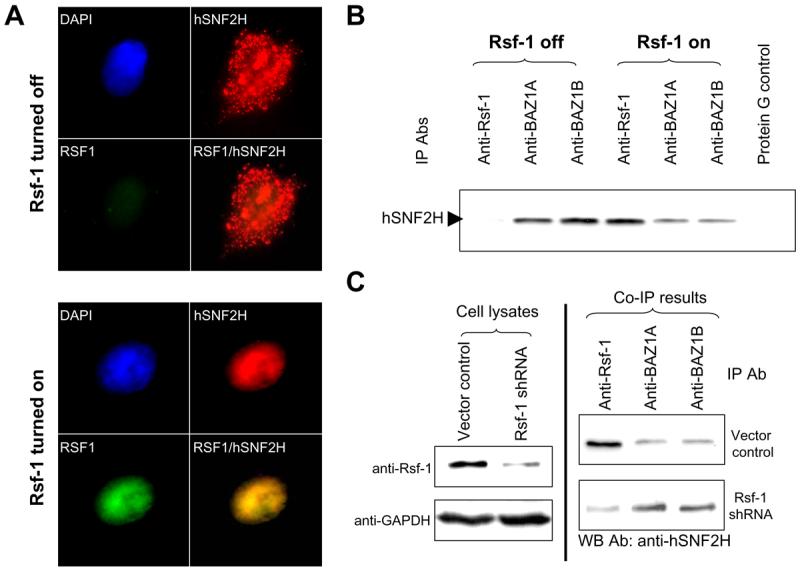
Analysis of interaction between hSNF2H and its binding partners. A: Immunofluorescence staining of Rsf-1 and hSNF2H proteins shows that RSf-1 co-localizes with hSNF2H in the nuclei. Rsf-1 inducible RK3E cells were transiently transfected with hSNF2H gene fused with an Xpress tag. Double immunofluorescence staining was performed to detect Rsf-1 (green fluorescence; anti-V5) and hSNF2H (red fluorescence; anti-Xpress) proteins, respectively. B: Immunoprecipitation was performed in SKOV3 cells stably transfected with a Tet-off inducible Rsf-1 expression construct. The protein level of hSNF2H that was co-immunoprecipitated with Rsf-1 was increased in Rsf-1 induced (Rsf-1 on) cells but its level co-immunoprecipitated with BAZ1A and BAZ1B significantly reduced as compared to the control SKOV3 cells without Rsf-1 induction (Rsf-1 off). Equal protein amount was used in immunoprecipitation for each antibody. C: Immunoprecipitation assays were performed to compare the binding capability between hSNF2H and different binding partners. Rsf-1 gene expression was knocked down by shRNA in Rsf-1 amplified OVCAR3 cells (left panel). The cell lysate of equal protein amount from Rsf-1 shRNA and control vector transfected cells was then immunoprecipitated with anti-Rsf-1, anti-BAZ1A and anti-BAZ1B antibodies (Abs), respectively (right panel). The immunoprecipitated complex was separated by SDS-PAGE and blotted with an anti-hSNF2H antibody to determine the amount of co-immunoprecipitated hSNF2H binding partners including Rsf-1, BAZ1A and BAZ1B.
