Figure 2. IL-12p70 produced by transfected MDDC is bioactive and enhances target-specific responses of NK cells.
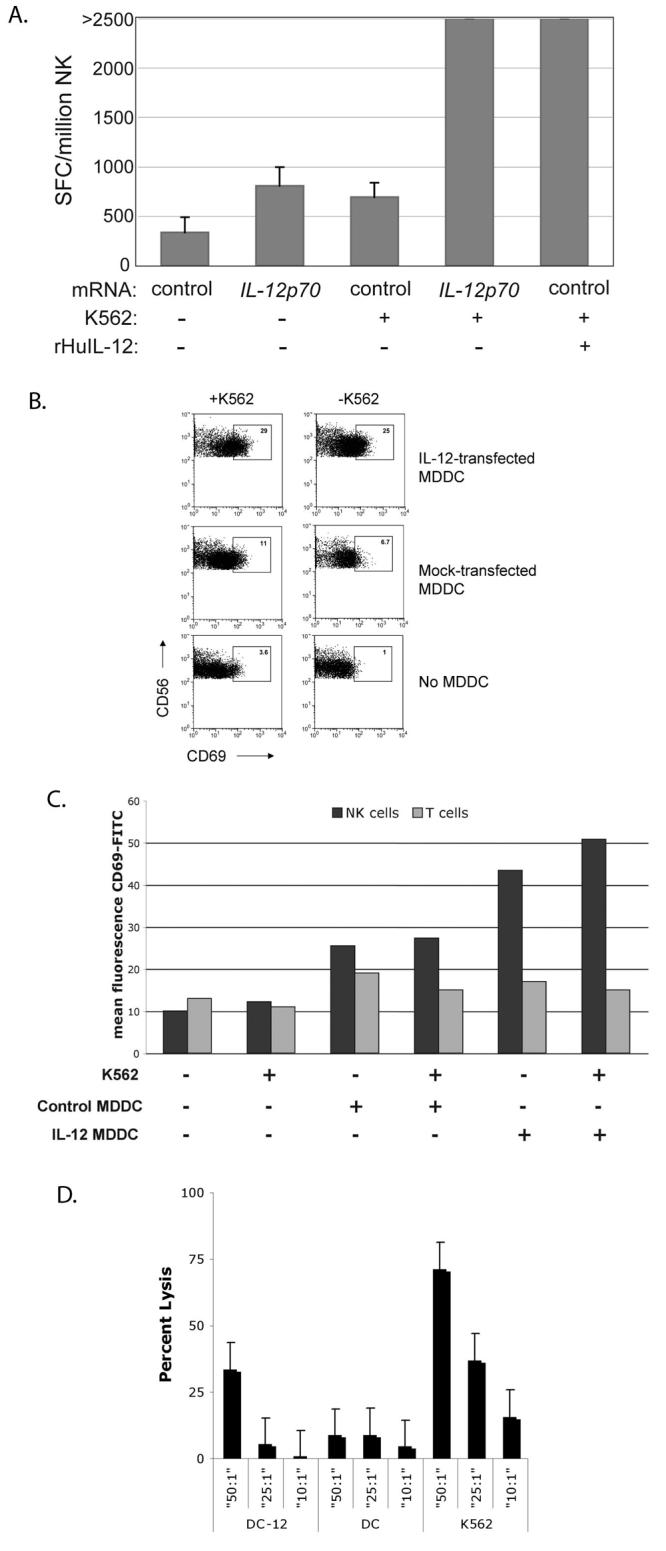
A. NK cells isolated from PBMC of a normal donor were incubated with autologous mock-transfected or IL-12p70-transfected MDDC in the presence or absence of class I-negative K562 targets. rHuIL-12 was added to co-cultures of NK cells and mock-transfected MDDC as an additional control. IFN-γ production, a measure of NK cell activation, was subsequently measured in an overnight ELISpot assay.
B. PBMC from a normal donor were co-cultured overnight with autologous IL-12p70-transfected MDDC in the presence or absence of K562 targets as shown. The degree of NK cell activation was determined by measuring CD69 expression on CD3negCD56+ lymphocytes with FACS. The percent CD69+ cells is shown within the gate.
C. Mean fluorescence intensity (MFI) of CD69 on CD3negCD56+ NK cells and CD3+CD56neg T cells. Enhancement of activation by IL-12p70 was specific for CD3negCD56+ NK cells, as there was no change in the activation state of CD3+CD56neg T cells. Results in this figure are a representation of three similar experiments.
D. IL-12 production by immature MDDCs increases the cytolytic activity of autologous NK cells. Immature MDDC were transfected with IL-12p70 or control mRNA, labeled with 51Cr and incubated with fresh autologous NK cells for four hours in a chromium release assay; untransfected K562 targets were used as a positive control for cytolytic activity, The percent lysis was calculated as [((sample count−spontaneous release)/(maximal release−spontaneous release))×100].
