Figure 6. Temporal dynamics of the activated αCaMKII-F89G during LTP in the Schaffer collateral CA1 pathway in transgenic mice.
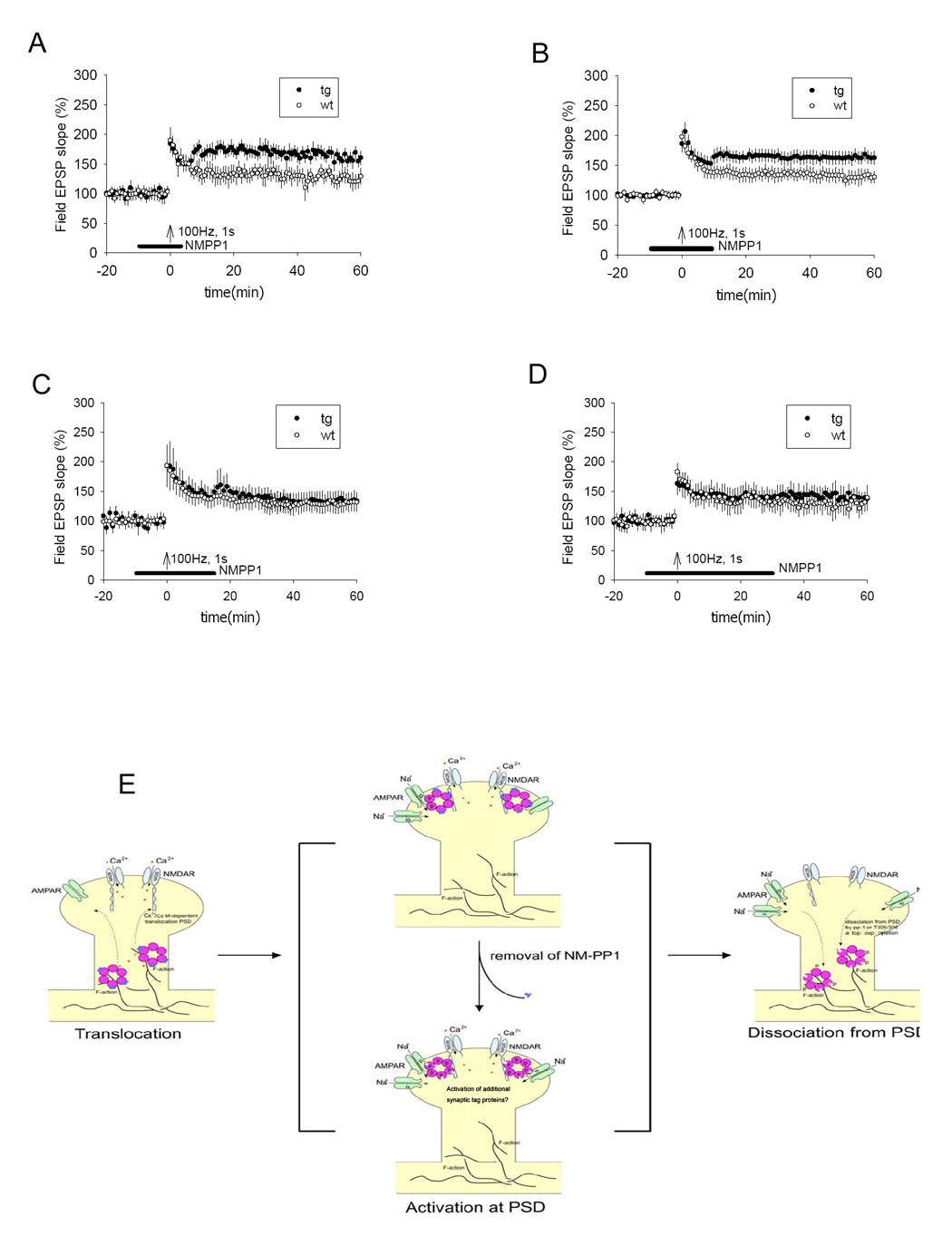
(A) LTP in transgenic slices was shifted to a higher level from the otherwise normal level when NM-PP1 was washed out 5 minutes after tetanus (p < 0.05, 8 transgenic mice, 10 slices; 8 control mice, 10 slices). (B) LTP in transgenic slices was also shifted to a higher level when NM-PP1 was washed out 10 minutes after tetanus (p < 0.05, 8 transgenic mice, 10 slices; 8 control mice, 10 slices). (C) No shift was observed when NM-PP1 was washed out 15 minutes after tetanus (8 transgenic mice, 11 slices; 8 control mice, 11 slices). (D) No shift was observed when NM-PP1 was washed away 30 minutes after tetanus (8 transgenic mice, 11 slices; 8 control mice, 11 slices). All values are expressed as mean ± S.E.M. ANOVA-Tukey was used to determine statistical difference in the magnitude of LTP from last 10 minutes block of recordings (51–60 minutes) between transgenic and control slices. The black bars indicate the duration of NM-PP1 treatment. (E) Schematic drawing illustrates the molecular dynamics of CaMII at activated synapses. During the Initiation phase, learning triggers the activation of the NMDA receptor which permits Ca2+ influx, leading to the binding of Ca2+/calmodulin to CaMKII, which initiates the dissociation of CaMKII from F-actin (through βCaMKII-mediated action) and then translocation of the complex to PSD. The αCaMKII-F89G-containing holoenzyme in the transgenic animals is capable of translocating to PSD in the presence of NM-PP1 inhibition because the translocation is an autophosphorylation-independent step. Once translocated to PSD via binding to NR2B C-terminal, the active CaMKII potentiates synapses and activates ‘synaptic tags’. During this PSD-associated phase, changes in CaMKII activity (such as unmasking the activated αCaMKII-F89G activity) will lead to reset the patterns of potentiated synapses in the network. The dissociation of CaMKII from PSD would make the synapse less vulnerable to the unmasking effect of αCaMKII-F89G activity. The dissociation may be controlled by the dephosphorylation on T286 by phosphatase 1, secondary phosphorylation at inhibitory phosphorylation sites of αCaMKII, and/or βCaMKII activity.
