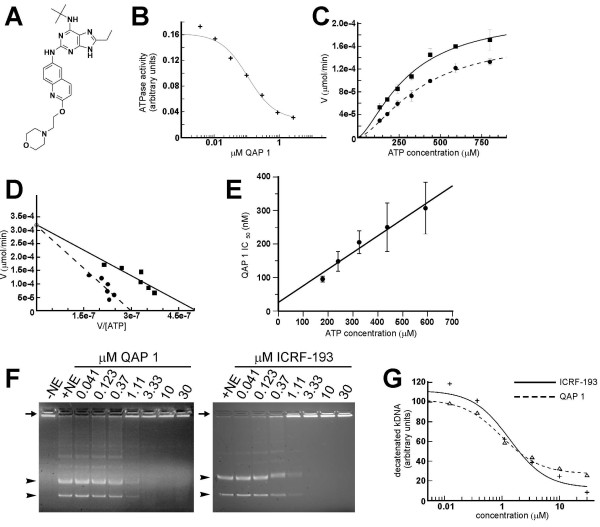
Figure 1.
Identification of QAP 1 as an ATP-competitive catalytic inhibitor of topoisomerase II. (A) Chemical structure of the substituted purine analogue QAP 1. (B) Concentration-dependent inhibition of topoisomerase II ATPase activity by QAP 1. (C, D) Kinetic analysis of DNA-dependent topoisomerase II ATPase activity with vehicle or QAP 1 (130 nM) showing product formation in dependence of substrate concentration (C) and Eadie-Hofstee plot (D). (E) Plot representing the IC50s determined in the presence of different ATP concentrations. (F) Inhibition of topoisomerase II-mediated DNA decatenation in vitro by QAP 1 as compared to ICRF-193. Assays were carried out with topoisomerase II from nuclear extracts (NE). The arrow marks the position of catenated kDNA substrate and the arrowheads designate the positions of decatenated kDNA topoisomerase II products, nicked circular and closed circular minicircles, respectively. (G) IC50 determination of the inhibition of DNA decatenation by QAP 1 and ICRF-193, respectively.