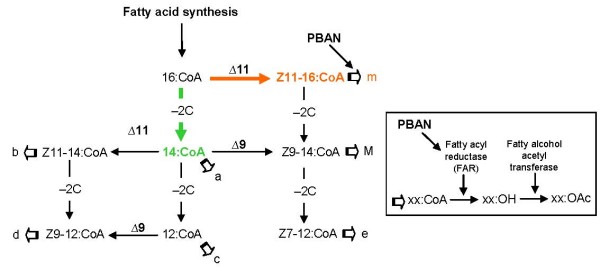
Figure 5.
Proposed pathways of the biosynthesis of the pheromone components in S. frugiperda (based on biosynthetic pathways described for other moth species by Jurenka, 2003). The interplay of desaturation and chain shortening of 16-, and 14-carbon acyl-CoA derivatives produce mono-unsaturated acyl-CoA precursors that are then reduced and acetylated to produce acetate esters. The number that follows Δ indicates the position of the double bond introduced by the desaturase into the acyl-CoA. -2C indicates chain-shortening by two carbons through β-oxidation. The order of desaturation and chain shortening results in different compounds. The open arrows stand for reduction and acetylation. The letters behind the open arrows stand for the pheromone compounds mentioned in all Tables and Figures.