Abstract
OBJECTIVE—Diabetes is associated with cognitive decline and dementia. However, the relationship between the degree of hyperglycemia and cognitive status remains unclear. This was explored using baseline cognitive measures collected in the ongoing Memory in Diabetes (MIND) substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
RESEARCH DESIGN AND METHODS—The relationship of A1C and fasting plasma glucose (FPG) levels to performance on four cognitive tests was assessed, adjusting for age and other determinants of cognitive status. The tests were the Digit Symbol Substitution Test (DSST), Mini Mental Status Examination (MMSE), Rey Auditory Verbal Learning Test, and Stroop Test.
RESULTS—A statistically significant age-adjusted association was observed between the A1C level and the score on all four cognitive tests. Specifically, a 1% higher A1C value was associated with a significant 1.75-point lower DSST score (95% CI −1.22 to −2.28; P < 0.0001), a 0.20-point lower MMSE score (−0.11 to −0.28; P < 0.0001), a 0.11-point lower memory score (−0.02 to −0.19, P = 0.0142), and a worse score (i.e., 0.75 s more) on the Stroop Test (1.31–0.19, P = 0.0094). The association between the DSST score and A1C persisted in all multiple linear regression models. FPG was not associated with test performance.
CONCLUSIONS—Higher A1C levels are associated with lower cognitive function in individuals with diabetes. The effect of glucose lowering on cognitive function will be determined by the ongoing ACCORD-MIND trial.
Mild cognitive impairment represents an important phase on the path from normal cognitive function to dementia. Affected individuals have measurable deficits in cognitive function that may affect their ability to master complex behaviors such as those required for diabetes self-care (1). Moreover, because mild cognitive impairment is more common than frank dementia, its potential population health impact is high. For example, the prevalence of mild cognitive impairment (i.e., predementia) in the Cardiovascular Health Study was 19% in individuals aged >65 years and 29% in those aged >85 years.
Diabetes is associated with premature mortality and is a risk factor for mild cognitive impairment and both vascular dementia (2–5) and Alzheimer's disease (2,6–8). Indeed, individuals with diabetes are ∼1.5 times more likely to experience cognitive decline and frank dementia than individuals without diabetes (9). Precise reasons for the high morbidity and mortality of diabetes remain unknown; however, many studies have demonstrated a link between many of the consequences of diabetes and the degree of hyperglycemia as measured by the A1C or glucose level. Emerging evidence suggests that a relationship between measures of short-term glucose control and cognitive function also exists. For example, in a cross-sectional analysis of 378 high-functioning individuals with diabetes, higher A1C but not fasting plasma glucose (FPG) levels were consistently associated with lower scores on two cognitive tests (10). Smaller studies reported a similar relationship with indexes of dysglycemia (11,12); nevertheless, details regarding such a relationship remain unclear.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is a randomized controlled trial of 10,251 individuals with established type 2 diabetes who have a high risk for cardiovascular disease (CVD) and whose screening A1C was ≥7.5%. It will determine whether therapeutic strategies targeting normoglycemia, normotension, and/or a normal lipid profile can reduce the rate of cardiovascular events more than standard therapeutic approaches in individuals with type 2 diabetes and either previous cardiovascular events or additional cardiovascular risk factors. The Memory in Diabetes (MIND) substudy of the ACCORD trial will determine whether these interventions reduce cognitive decline and structural brain changes in a subset of 2,977 randomized individuals from sites that participated in the MIND substudy. Baseline data from the MIND substudy provide a unique opportunity to assess the cross-sectional relationship between cognitive function and two different measures of glycemia: A1C and FPG.
RESEARCH DESIGN AND METHODS
The design of the ACCORD-MIND trial was described elsewhere (13). In brief, six of seven ACCORD clinical networks in the U.S. and Canada comprising 52 of 77 sites participated in the ACCORD-MIND trial.
ACCORD participants aged >55 years who were fluent in English or Spanish and willing to participate and who had been randomized into the main ACCORD trial for <45 days were invited to participate in the MIND substudy. After signing a consent form, eligible participants completed a 30-min battery of cognitive tests. To ensure that participants were not hypoglycemic at the time of cognitive testing, the tests were generally administered after breakfast, and a capillary glucose level was measured before testing. If the result was <60 mg/dl (3.3 mmol/l), a snack was given, and the capillary glucose value was measured again within 15 min; if the repeat glucose level at that time was still <60 mg/dl, the test was rebooked for a different day. The battery was administered and scored by certified technicians (either in English or Spanish), and the data were entered centrally at the ACCORD-MIND coordinating center. The study protocol was approved by the research ethics board of each participating center, and all participants provided written informed consent.
Measures of glycemic status
Baseline biochemical characteristics of MIND participants were measured in the central laboratory of the ACCORD main trial. A1C was measured by a Tosah G7 automated high performance liquid chromatograph (in a laboratory with National Glycohemoglobin Standardization Program level I certification for traceability to the Diabetes Control and Complications Trial reference); FPG was measured enzymatically on an Hitachi 917 autoanalyzer.
Cognitive testing
Cognitive tests that measured performance in specific domains of interest were chosen because they were standardized, widely used, had well-established norms, and could be administered by nonneuropsychologists. Cognitive training session and certification of MIND technicians were conducted by MIND coordinating center data quality assurance staff who were trained and supervised by MIND investigators, as described previously (14).
Recertification of test performance was repeated 6 months after the initial certification and annually thereafter. Thus, a planned review of the test administrators’ performance, which included a review of the test session recorded via audiotape and examination of actual test materials and scores, provided ongoing quality assurance of the cognitive data. Cognitive test administrators were provided written feedback on their performance and were retrained as needed. Because of the significant number of Spanish-speaking participants, validated translations were used for the Mini Mental Status Examination (MMSE) and Rey Auditory Verbal Learning Test (RAVLT) (15). Verified Spanish versions were created for the other tests by the Columbia University Hispanic Research and Recruitment Center team through translation and back-translation.
The tests have been described previously (13). In brief, the Digit Symbol Substitution Test (DSST) is a subtest of the Wechsler Adult Intelligence Scale (3rd edition), which assesses a wide array of cognitive domains, most prominently visual motor speed, capacity for learning, sustained attention, and working memory. It has been used extensively to measure cognitive function in cognitively intact individuals, and its score is well correlated with measures of physical function and future cognitive decline (16). The range of scores is 0 to 133, with increasing scores indicating better performance.
The MMSE is a screening tool for detecting changes in cognitive skills (17). It can also identify changes in cognitive function for elderly individuals without dementia and may identify individuals in the prodromal phase of dementia. The range of scores is 0 to 30, with increasing scores indicating better performance.
The RAVLT assesses the ability to memorize and to retrieve words (verbal memory). The RAVLT has been used extensively in epidemiological research and has been found to be sensitive to neurological impairment in a wide variety of patients.
The Stroop Test evaluates the ability to view complex visual stimuli and to respond to one stimulus dimension while suppressing the response to another dimension, an “executive” skill largely attributed to frontal lobe function.
Measures of confounding variables and covariates
We adjusted the analyses for several factors that may confound the association of the glycemic status measures and cognitive function, including 1) prevalent CVD, defined as a history of myocardial infarction, angina with ischemic changes on a graded exercise test or positive imaging, previous coronary revascularization procedures, or stroke; 2) hyperlipidemia, defined as use of any lipid-lowering agent or an untreated LDL cholesterol level >130 mg/dl (3.38 mmol/l); 3) hypertension, defined as either a history of hypertension or use of any antihypertensive agents; 4) alcohol consumption, defined as more than three drinks per week; 5) neuropathy, defined as either a history of neuropathy or absent ankle reflexes or vibration sense for either foot; 6) education, divided into three categories: no high school education, high school education only, or college education or more; and 7) depression, defined as either a history of depression or a score of 10 or higher on the Physicians Health Questionnaire (PHQ) 9, a depressive symptoms screening instrument. Other biochemical covariates measured in the central ACCORD laboratory included urine albumin, measured by the Dade Behring reagent on a Behring nephelometer (BNII); urine creatinine, measured by the Roche reagent on a Hitachi 917 chemistry autoanalyzer; and lipids, measured on a Hitachi 917 autoanalyzer using methods standardized to the Centers for Disease Control and Prevention reference methods.
Statistical analysis
The relationships between each of the four measures of cognitive status (i.e., the dependent variables), A1C, FPG, and the confounding and covarying variables described above were assessed with age-adjusted linear regression using the raw scores. Age adjustment was used because age may confound the relationship between dysglycemia and cognitive function.
Multiple linear regression was used to estimate the independent relationship between either A1C or FPG and each of the cognitive measures after controlling for 1) age, sex, education, and depression (model 1); 2) model 1 variables plus diabetes duration (model 2); 3) model 1 variables plus diabetes duration, race, and language (model 3); 4) model 1 variables plus a history of CVD (model 4); 5) model 1 variables plus stroke (model 5); and 6) model 1 variables plus all of the independent variables assessed in the simple linear regressions in Table 2 (model 6). For categorical independent variables coded as 0 or 1 indicator variables (e.g., hypertension), the β-coefficient represents the difference in predicted scores between those with and without the variable. For continuous independent variables (e.g., A1C), the β-coefficient represents the difference in predicted scores for every 1-unit difference (e.g., 1% for A1C) in the independent variable. The calculated R2 for each model indicates the percentage of variability in cognitive test score results explained by the model.
Table 2.
β-coefficients for the age-adjusted relationship between cognitive test scores, glycemic status, and other variables: ACCORD-MIND
| Variable | DSST | MMSE | Memory score | Stroop |
|---|---|---|---|---|
| Female sex | 1.79‖ | −0.27‖ | 1.30§ | 0.41 |
| Diabetes duration (years) | −0.26§ | 0.14‖ | −0.01 | −0.01 |
| CVD* | −1.98‖ | 0.12 | −0.48§ | 1.43‖ |
| Stroke | −8.18§ | −0.69‖ | −0.86§ | 4.53‖ |
| Nonstroke CVD | −0.08 | 0.32‖ | −0.31‖ | 0.40 |
| Hyperlipidemia† | 1.95‖ | 0.02 | −0.12 | −1.22 |
| Hypertension or blood pressure drugs | −1.78‖ | −0.11 | −0.14 | 0.37 |
| Current smoker | −2.75‖ | −0.29‖ | −0.46‖ | 0.38 |
| >3 drinks/week | 4.80§ | 0.76§ | −0.01 | −2.81‖ |
| Education | ||||
| < high school | −22.51§ | −3.24§ | −1.89§ | 12.30§ |
| High school | −10.63§ | −1.4§ | −0.89§ | 6.63§ |
| Some college | −5.2§ | −0.6§ | −0.41‖ | 3.86§ |
| Language | −25.97§ | −2.7§ | −1.51§ | 10.27§ |
| Race (Nonwhite) | −13.39§ | −1.92§ | −0.83§ | 6.71§ |
| Vitrectomy | −7.78‖ | 0.49 | 0.50 | 7.11 |
| Neuropathy‡ | 1.84‖ | 0.41§ | 0.17 | −0.82 |
| Depression or PHQ 9 score ≥10 | −1.04 | −0.34‖ | −0.05 | 0.54 |
| Living alone | 0.21 | 0.07 | 0.27‖ | 1.19 |
| BMI (kg/m2) | 0.20‖ | 0.04§ | 0.04§ | 0.04 |
| Urine albumin-to-creatinine ratio | −3.06§ | −0.11 | −0.20 | 1.29 |
| A1C (%) | −1.75§ | −0.20§ | −0.11‖ | 0.75‖ |
| Fasting plasma glucose (mmol/l) | 0.0057 | 0.0006 | 0.00006 | −0.0055 |
Myocardial infarction, angina with ischemic changes on graded exercise test or positive imaging, coronary revascularization procedures, or stroke.
Taking lipid-lowering medication or an untreated LDL cholesterol level >130 mg/dl (3.38 mmol/l).
History of neuropathy or absent ankle reflexes or vibration perception at great toe for either foot.
P ≤ 0.0001;
P ≤ 0.05.
RESULTS
As noted in Table 1, the 2,977 trial participants had mean age of 62.5 years, mean A1C of 8.3%, and mean FPG of 175.5 mg/dl (9.75 mmol/l). A total of 1,388 (47%) were women, 718 (24%) reported previous CVD that was not stroke-related, 151 (5%) reported a previous stroke, and 2,578 (87%) reported previous hypertension; 392 (13%) did not complete high school and 980 (33%) had either a history of depression or a PHQ 9 score consistent with some depression. These baseline characteristics are similar to those reported for the overall ACCORD trial (18).
Table 1.
Baseline characteristics of ACCORD-MIND participants
| Variable | Result |
|---|---|
| n | 2,977 |
| Female sex (%) | 1,388 (47) |
| Age (years) | 62.5 ± 5.8 |
| Diabetes duration (years) | 10.4 ± 7.3 |
| Mean BMI (kg/m2) | 33.0 ± 5.4 |
| Mean urine albumin-to-creatinine ratio | 0.092 ± 0.404 |
| Previous cardiovascular disease (%)* | 869 (29) |
| Stroke (%) | 151 (5) |
| Nonstroke (%) | 718 (24) |
| Hyperlipidemia (%)† | 2,426 (82) |
| Previous hypertension or use of blood pressure drugs (%) | 2,578 (87) |
| Current smoker (%) | 352 (12) |
| >3 drinks/week (%) | 232 (8) |
| Education | |
| Not a high school graduate (%) | 392 (13) |
| Just high school (%) | 769 (26) |
| Some college or technical school (%) | 1,027 (35) |
| College graduate or more (%) | 789 (27) |
| Ethnicity | |
| Hispanic (%) | 213 (7) |
| Non-Hispanic white (%) | 2,074 (70) |
| African American/African Canadian (%) | 478 (16) |
| Asian (%) | 67 (2) |
| American Indian/Alaska Native (%) | 65 (2) |
| Other (%) | 80 (2.6) |
| Vitrectomy (%) | 15 (0.5) |
| Neuropathy (%)‡ | 1,472 (50) |
| Past or current depression or PHQ 9 score ≥10 (%) | 980 (33) |
| Living alone (%) | 654 (22) |
| Cognitive testing in Spanish | 63 ± 2.1 |
| DSST score | 53 (42–63) |
| MMSE score | 28 (26–29) |
| Memory score | 7.4 (5.7–9.3) |
| Stroop Test score (s) | 29 (21–38) |
| A1C (%) | 8.3 ± 1.1 |
| FPG (mg/dl) | 175.5 ± 55 |
Data are means ± SD, n (%), or median (25th–75th percentile). The range of scores for the ACCORD participants is DSST 2–97, MMSE 12–30, memory 0.9–14.5, and Stroop −10 to 171.
Myocardial infarction, angina with ischemic changes on graded exercise test or positive imaging, coronary revascularization procedures, or stroke.
Taking lipid-lowering medication or an untreated LDL cholesterol level >130 mg/dl (3.38 mmol/l).
History of neuropathy or absent ankle reflexes or vibration perception at great toe for either foot.
Relationship between measures of glycemia and cognitive test scores
A1C.
A statistically significant age-adjusted association was observed between the A1C level and the score on all four cognitive tests (Table 2). Specifically, a 1% higher A1C value was associated with a 1.75-point lower DSST score (95% CI −1.22 to −2.28; P < 0.0001), a 0.20-point lower MMSE score (95% CI −0.11 to −0.28; P < 0.0001), a 0.11-point lower memory score (95% CI −0.02 to −0.19, P = 0.0142), and a worse score (i.e., 0.75 s more) on the Stroop Test (95% CI 1.31–0.19, P = 0.0094).
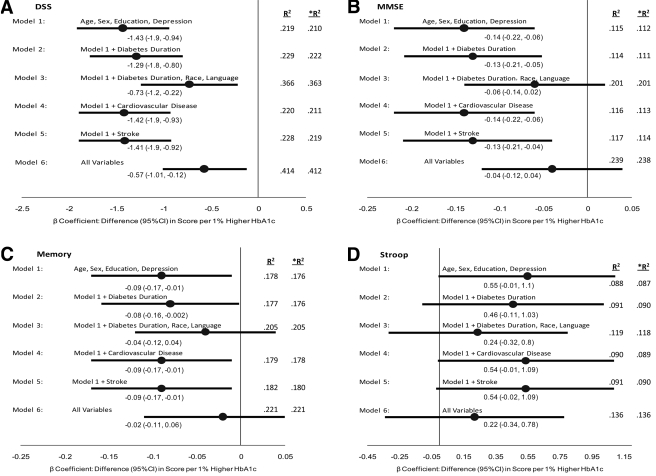
As noted in Fig. 1, a significant relationship between a higher A1C and lower DSST score persisted in all six models, with the weakest relationship noted after accounting for race and language. A similar significant relationship was noted for the adjusted MMSE and memory scores in some of the models; however, higher A1C levels were not significantly associated with higher Stroop Test scores after adjustment for the variables in any model. Thus, after adjustment for age, sex, education, and depression score (model 1, Fig. 1A–C), a 1% higher A1C was associated with a 1.43-point (95% CI −0.94 to −1.92; P < 0.0001) lower DSST score, a 0.14-point (−0.06 to −0.22; P = 0.001) lower MMSE score, and a 0.09-point (−0.01 to −0.17; P = 0.02) lower memory score.
Figure 1.
The associations between a 1% increase in A1C (percentage) and test scores on four different measures of cognitive function (and their 95% CIs) after adjustment for different baseline characteristics are shown. The sixth model includes all of the variables noted in Table 1. A: DSST. B: MMSE. C: Memory score (Rey Auditory Verbal Learning Test). D: Stroop Test. R2 is the percentage of the variance for each cognitive test explained by each model (including the term for A1C), and *R2 is the percentage of the variance explained by each model without the A1C term included.
Figure 1 also shows R2 values for the adjusted models both with and without the A1C term. The small differences in the two R2 values for each model where A1C is associated with the cognitive test indicates that A1C levels, although statistically significant, only explain a small additional amount of the variability in cognitive test scores compared with that explained by the model excluding the A1C term.
FPG.
Unlike for A1C, no significant relationship between the FPG and any of the cognitive tests was observed in the age-adjusted or multiple regression analyses (Table 2).
CONCLUSIONS
This analysis of ∼3,000 individuals with established type 2 diabetes demonstrates a clear age-adjusted inverse relationship between cognitive function and the degree of chronic hyperglycemia as measured by the A1C level. The observed effect of a 1% change in A1C on tests scores is clearly small; nevertheless, such an effect may be clinically important. For example, in the same sample every 1-year increase in age was associated with a 0.7-point decrease in DSST score (data not shown). Therefore, the 1- to 1.5-point difference in DSST per 1% higher A1C corresponds to an age difference of up to 2 years. Moreover, several recent studies demonstrated the clinical importance of this difference. Thus, a cross-sectional study in older healthy individuals reported that a 3-point difference in DSST score was associated with lower scores on physical performance tests (16), and in a 3-year follow-up study a 1-point difference in baseline DSST score significantly increased the risk for the development of Alzheimer's disease among individuals with minimal cognitive impairment (19). Finally, during a mean follow-up of 3.3 years, a 1-point difference in baseline DSST score was associated with a 3% increase in the risk for dementia in community dwellers aged >70 years (20).
The relationship between the A1C level and cognition was attenuated after adjustment for other factors associated with cognitive function in some of the models but remained significant for the DSST score in every adjusted model. These findings suggest that much, but not all, of the relationship between A1C and cognitive function may be explained by risk factors other than A1C. Thus, the small increment in the R2 value (Fig. 1) attributable to addition of the A1C term to the multivariable models (which reflects the degree to which the model accounts for the cognitive test score) suggests that A1C is not the crucial determinant of cognitive score after consideration of these other factors and particularly those related to ethnicity/language. However, these other factors are mostly not modifiable, whereas A1C levels can be changed with therapy. This evidence for a small additional significant effect of A1C on cognitive test scores therefore supports (but clearly does not prove) the hypothesis that lowering A1C may have an impact on these scores.
Taken together, these analyses extend previous reports of a link between cognitive decline and diabetes and are consistent with the hypothesis of a progressive relationship between the degree of chronic hyperglycemia and cognitive dysfunction.
The fact that optimal diabetes care requires affected patients to make therapeutic decisions based on information that they collect and process highlights the clinical significance of this finding. Finally, the absence of a clear relationship between FPG and these tests may be due to the fact that FPG is not as reliable as A1C as a measure of the underlying chronic glycemic status.
A number of possibilities may explain these findings. Because higher glucose levels are associated with a higher prevalence of cardiovascular risk factors and CVD, the relationship with cognitive dysfunction may be mediated through CVD. The fact that this relationship is not attenuated by adjusting for CVD reduces but does not completely eliminate this possibility. It is also possible that chronic exposure of the brain to high levels of glucose may accelerate cognitive decline. Indeed, postmortem studies of senile plaques from the brains of individuals with Alzheimer's disease demonstrate metabolic oxidation products associated with hyperglycemia (21,22).
A third possibility is related to the fact that higher A1C levels imply insufficient action or effect of insulin due to insufficient secretion, activity, or both. There are many insulin receptors in the brain. Some have a role in glucose transport, but many are thought to have a function in cognitive processes. Several observations suggest that cognitive decline is a consequence of reduced insulin action in the brain. In individuals without diabetes, worse glucoregulation (as measured by a glucose tolerance test) was associated with worse outcomes on cognitive assessment, especially in elderly individuals. Individuals with Alzheimer's disease also have less efficient glucoregulation than unaffected individuals (23), and exposure of individuals with Alzheimer's disease to a euglycemic-hyperinsulinemic clamp improved cognitive function, whereas exposure to a euinsulinemic-hyperglycemic clamp had no effect (23,24).
There are limitations to this study. First, because the analyses were cross-sectional, it is not possible to make any temporal or causal inferences regarding relationships. Second, cognitive tests were administered to individuals of several ethnic groups and in two languages, thus increasing the variability of the measurement. Third, the ACCORD trial excluded individuals whose most recent A1C was <7.5% or >11% and those who were deemed unable to participate in their diabetes management; these results may therefore not apply to individuals with lower or higher A1C levels or significant cognitive impairment. Fourth, exclusion of individuals with lower A1C levels means that the relationship between A1C and cognition is studied over a narrow range of A1C levels. This reduces the ability to detect a relationship, and thus the link between A1C and cognition may have been underestimated. However, this large sample of individuals with diabetes is well powered to study the association of A1C levels and cognition.
In summary, this cross-sectional analysis illustrates that chronic hyperglycemia appears to be independently associated with cognitive function in individuals with diabetes. It also raises the hypothesis that strategies to lower A1C levels or prevent their rise may favorably affect cognitive function. Such a hypothesis can only be tested in prospective studies, such as the ongoing ACCORD-MIND trial.
Acknowledgments
This work was supported by contract-N01-HC95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA Y1-HC-9035, and IAA Y1-HC-1010 from the National Heart, Lung, and Blood Institute (NHLBI), with additional support from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Eye Institute, Bethesda, MD; the National Institute on Aging (NIA); and the Centers for Disease Control and Prevention. This study was also supported by Grant NIHNHLBI-HC-99-16 from the NIA/NHLBI, by the National Institutes of Health–funded General Clinical Research Centers, and by the Intramural Research Program at the NIA.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
APPENDIX
The Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators are the following. Executive Committee: Lenore J. Launer, R. Nick Bryan, Mike E. Miller, Jeff D. Williamson. Canadian Clinical Center Network (CCN): McMaster University and Hamilton Health Sciences, Hamilton, ON, Canada: Hertzel Gerstein, MD, MSc; Tali Cukierman-Yaffee, MD, MSc. Western CCN: University of Washington, Seattle, WA: Mark D. Sullivan, MD, PhD. Minnesota-Iowa CCN: Berman Center for Outcomes and Clinical Research, Minneapolis, MN: Anne Murray, MD, MSc. Ohio-Michigan CCN: Case Western Reserve University, Cleveland, OH: Karen R. Horowitz, MD. Northeastern CCN: Columbia University College of Physicians and Surgeons, New York, NY: Ronald M. Lazar, PhD. Southeastern CCN: Wake Forest University School of Medicine, Winston-Salem, NC: Jingzhong Ding, MD, PhD. Coordinating Center: Wake Forest University School of Medicine, Winston-Salem, NC: Jeff D. Williamson, MD, MHS; Laura H. Coker, PhD; Michael E. Miller, PhD. MRI Quality Control: University of Pennsylvania, Philadelphia, PA: R. Nick Bryan, MD, PhD. Project Office: National Institute on Aging, National Institute of Health, Bethesda, MD: Lenore J. Launer, PhD.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Glasgow RE, Fisher L, Skaff M, Mullan J, Toobert DJ: Problem solving and diabetes self-management: investigation in a large, multiracial sample. Diabetes Care 30:33–37, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G: Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc 51:410–414, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R: Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154:635–641, 2001 [DOI] [PubMed] [Google Scholar]
- 4.MacKnight C, Rockwood K, Awalt E, McDowell I: Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord 14:77–83, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia AS: Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51:1256–1262, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA: Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61:661–666, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ott A, Stolk RP, van HF, Pols HA, Hofman A, Breteler MM: Diabetes mellitus and the risk of dementia: the Rotterdam Study [see comment]. Neurology 53:1937–1942, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ: Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145:301–308, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Cukierman T, Gerstein HC, Williamson JD: Cognitive decline and dementia in diabetes—a systematic overview of prospective observational studies. Diabetologia 42:2460–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Shorr RI, de Rekenkeire N, Resnick HE, Yaffe K, Somes GW, Kanaya AM, Simonsick EM, Newman AB, Harris TB: Glycemia and cognitive function in older adults using glucose-lowering drugs. J Nutr Health Aging 10:297–301, 2006 [PubMed] [Google Scholar]
- 11.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL: Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 28:71–77, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW: Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 29:345–351, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Williamson JD, Miller ME, Bryan N, Lazar RM, Coker LH, Johnson J, Cukierman T, Horowitz KR, Murray A, Launer LJ, ACCORD Study Group: The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study(ACCORD-MIND): Rationale, design and methods. Am J Cardiol 99(Suppl.): S112–S112, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Coker LH, Felton D, Andrews LA, Tooze J, Lovato J, Lovato L, Woolard N, Williamson J, Lazar RM, Miller M, Launer L: Quality assurance of cognitive outcomes in a randomized clinical trial; the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Trial. Clin Trials 5:399–400, 2008 [Google Scholar]
- 15.Mungas D, Reed BR, Marshall SC, Gonzalez HM: Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 14:209–223, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman AB: Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology 24:8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Tombaugh TN, McIntyre NJ: The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc 40:922–935, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probsfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559 [DOI] [PMC free article] [PubMed]
- 19.Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B: Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology 69:1859–1867, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB: Within-person across neuropsychological test variability and incident dementia. J R Soc Med 87:619–621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K: Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer's disease and aged neurons. Biochem Biophys Res Commun 236:327–332, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Vlassara H, Bucala R, Striker L: Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest 70:138–151, 1994 [PubMed] [Google Scholar]
- 23.Watson GS, Craft S: Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer's disease. Eur J Pharmacol 490:97–113, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Messier C: Glucose improvement of memory: a review. Eur J Pharmacol 490:33–57, 2004 [DOI] [PubMed] [Google Scholar]