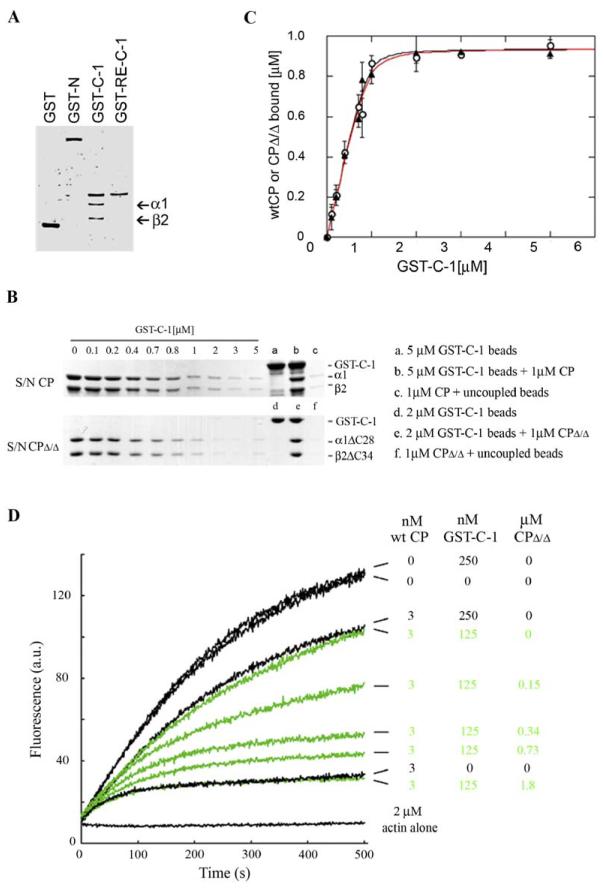
Figure 3.
C-1 Binds Directly to CP
(A) Pure CP is pelleted by GST-C-1; binding to CP is decreased by the R993E mutation. GST or mCARMIL fusion proteins, GST-N-mCARMIL, GST-C-1, or GST-R993E-C-1 (each ∼500 nM) were incubated with glutathionesepharose beads, washed, and then incubated with CP, a heterodimer of α1 and β2 (100 nM). After washing, the bound protein was eluted with SDS buffer, separated on 10% SDS-PAGE, and stained with silver.
(B and C) GST-C-1 binds to wt CP and the CPΔ/Δ mutant with similar affinity. The ability of glutathione-sepharose-coupled GST-C-1 to pull down pure CP was determined by loss of CP from the supernatant. The CP concentration was constant at 1 μM, and increasing amounts of beads, providing a total concentration of GST-C1 up to 5 μM, were added. (B) shows representative Tris-SDS-polyacrylamide gels (12% acrylamide) illustrating the GST-C1 concentration-dependent depletion of CP or CPΔ/Δ from the supernatant (S/N). Controls for GST-C-1, wt CP added, and wt CP pelleted with uncoupled beads are shown in lanes labeled a–c, and for CPΔ/Δ in lanes labeled d–f. (C) is a plot of the concentration of bound CP (open circles) or CPΔ/Δ (filled triangles) versus the concentration of immobilized GST-C-1. Values are the mean ± SEM, n = 3. The data were least squares fit to equation 1. The difference is not statistically significant (p > 0.7).
(D) CPΔ/Δ mutant competes with wt CP for GST-C-1. Actin polymerization was followed from pyrenylactin fluorescence (a.u.) as a function of time (s) after nucleation by seeds in the presence of wt CP (3 nM) in the absence or presence of GST-C-1 (250 or 125 nM), and in the absence or presence of various concentrations of CPΔ/Δ. CPΔ/Δ at high concentrations completely relieved the GSTC-1-mediated inhibition of CP’s capping activity. Actin was used at 2 μM (5.8% pyrene labeled).