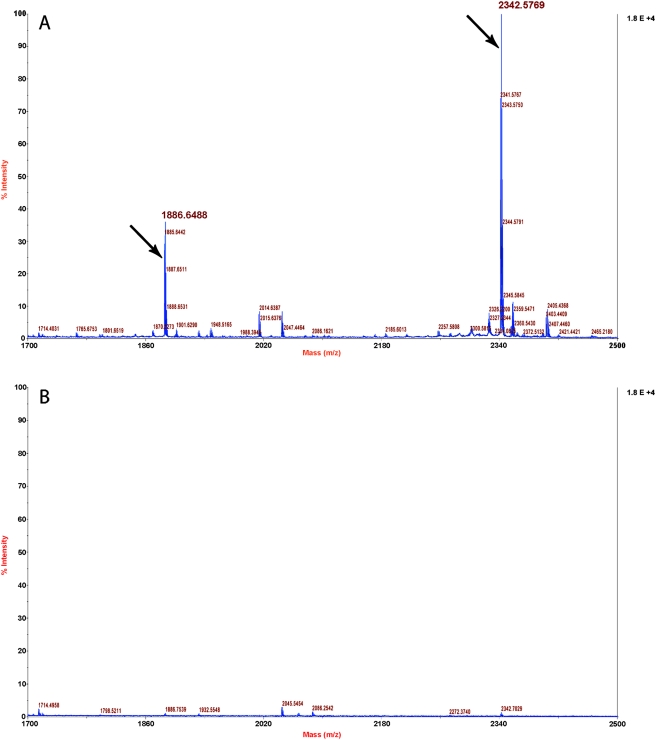
Figure 7. Mass spectrometric detection of intact BMAP-18 peptide after immunoenrichment from human plasma by anti-BMAP antibodies.
MALDI-TOF mass spectrometry was used to determine the peptide mass spectra of eluates from Dynabeads used to capture antibody-BMAP-18 complexes from human plasma. Panel A shows the spectrum obtained from 50 µL of human plasma spiked with 1.0 µg of BMAP-18 peptide after capture of BMAP-18 with affinity-purified anti-BMAP-18 antibody. Panel B shows the spectrum obtained when no peptide was spiked into the plasma. This negative control reveals that no off-target binding occurred. In Panel A the average mass is the peak indicated by the arrow at 2342.57 m/z. A smaller peak at 1886.64 m/z was also seen and is likely a degradation product of BMAP-18. Both Panel A and Panel B show accumulated spectra. This experiment was repeated twice and similar results were observed.