Abstract
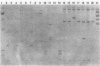
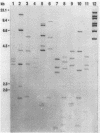
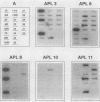
Beginning in 1990, gram-positive rods resembling Actinomyces pyogenes were found with increasing frequency in mixed cultures from various infectious processes, most of them from patients with otitis, empyema, pilonidal cysts, perianal abscesses, and decubitus ulcers. Ribotyping and hybridization showed that these gram-positive rods could be divided into five groups not related to known Actinomyces species. Biochemical markers for reliable differentiation into these groups, however, could not be found. Therefore, naming new species is not warranted unless parameters are discovered that allow identification without DNA hybridization. These gram-positive rods have been isolated only in mixed cultures with anaerobes, Staphylococcus aureus, Streptococcus "milleri," enterococci, and gram-negative rods. Their exact role in these possibly synergistic infections needs further investigation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnham M. Actinomyces pyogenes bacteraemia in a patient with carcinoma of the colon. J Infect. 1988 Nov;17(3):231–234. doi: 10.1016/s0163-4453(88)96522-x. [DOI] [PubMed] [Google Scholar]
- Bernard K. A., Bellefeuille M., Ewan E. P. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991 Jan;29(1):83–89. doi: 10.1128/jcm.29.1.83-89.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander M. A., Jousimies-Somer H. R. Evaluation of the RapID ANA II and API ZYM systems for identification of Actinomyces species from clinical specimens. J Clin Microbiol. 1992 Dec;30(12):3112–3116. doi: 10.1128/jcm.30.12.3112-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlosta E. M., Richards G. K., Wagner E., Holland J. F. An opportunistic infection with Corynebacterium pyogenes producing empyema. Am J Clin Pathol. 1970 Feb;53(2):167–170. doi: 10.1093/ajcp/53.2.167. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Jones D., Kroppenstedt R. M., Schleifer K. H. Chemical studies as a guide to the classification of Corynebacterium pyogenes and "Corynebacterium haemolyticum". J Gen Microbiol. 1982 Feb;128(2):335–341. doi: 10.1099/00221287-128-2-335. [DOI] [PubMed] [Google Scholar]
- Coyle M. B., Lipsky B. A. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev. 1990 Jul;3(3):227–246. doi: 10.1128/cmr.3.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent V. E., Williams R. A. Actinomyces denticolens Dent & Williams sp. nov: a new species from the dental plaque of cattle. J Appl Bacteriol. 1984 Apr;56(2):183–192. doi: 10.1111/j.1365-2672.1984.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Freney J., Duperron M. T., Courtier C., Hansen W., Allard F., Boeufgras J. M., Monget D., Fleurette J. Evaluation of API Coryne in comparison with conventional methods for identifying coryneform bacteria. J Clin Microbiol. 1991 Jan;29(1):38–41. doi: 10.1128/jcm.29.1.38-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrn-Hansen B., Frederiksen W. Human infections with Actinomyces pyogenes (Corynebacterium pyogenes). Diagn Microbiol Infect Dis. 1992 May-Jun;15(4):349–354. doi: 10.1016/0732-8893(92)90022-l. [DOI] [PubMed] [Google Scholar]
- Gavin S. E., Leonard R. B., Briselden A. M., Coyle M. B. Evaluation of the rapid CORYNE identification system for Corynebacterium species and other coryneforms. J Clin Microbiol. 1992 Jul;30(7):1692–1695. doi: 10.1128/jcm.30.7.1692-1695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem F. M., Ridpath A. C., Moore W. E., Moore L. V. Identification of Clostridium botulinum, Clostridium argentinense, and related organisms by cellular fatty acid analysis. J Clin Microbiol. 1991 Jun;29(6):1114–1124. doi: 10.1128/jcm.29.6.1114-1124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Moore L. V., Kaneko B., Moore W. E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990 Jul;40(3):273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Howell A. W., Maher L. A. Quantitative antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E-test. J Clin Microbiol. 1991 Jan;29(1):109–114. doi: 10.1128/jcm.29.1.109-114.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrajaras R., Tagami H. Corynebacterium pyogenes. Its pathogenic mechanism in epidemic leg ulcers in Thailand. Int J Dermatol. 1987 Jan-Feb;26(1):45–50. doi: 10.1111/j.1365-4362.1987.tb04575.x. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Blobel H. Comparative studies on Actinomyces pyogenes and Arcanobacterium haemolyticum. Med Microbiol Immunol. 1988;177(2):109–114. doi: 10.1007/BF00189532. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Blobel H. Tentative identification of Actinomyces pyogenes with antisera against group G streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Sep;262(3):357–360. doi: 10.1016/s0176-6724(86)80008-6. [DOI] [PubMed] [Google Scholar]
- Madsen M., Høi Sørensen G., Aalbaek B., Hansen J. W., Bjørn H. Summer mastitis in heifers: studies on the seasonal occurrence of Actinomyces pyogenes, Peptostreptococcus indolicus and Bacteroidaceae in clinically healthy cattle in Denmark. Vet Microbiol. 1992 Feb;30(2-3):243–255. doi: 10.1016/0378-1135(92)90118-d. [DOI] [PubMed] [Google Scholar]
- Martinetti G., Altwegg M. rRNA gene restriction patterns and plasmid analysis as a tool for typing Salmonella enteritidis. Res Microbiol. 1990 Nov-Dec;141(9):1151–1162. doi: 10.1016/0923-2508(90)90088-8. [DOI] [PubMed] [Google Scholar]
- Morris A., Guild I. Endocarditis due to Corynebacterium pseudodiphtheriticum: five case reports, review, and antibiotic susceptibilities of nine strains. Rev Infect Dis. 1991 Sep-Oct;13(5):887–892. doi: 10.1093/clinids/13.5.887. [DOI] [PubMed] [Google Scholar]
- Pitcher D., Soto A., Soriano F., Valero-Guillén P. Classification of coryneform bacteria associated with human urinary tract infection (group D2) as Corynebacterium urealyticum sp. nov. Int J Syst Bacteriol. 1992 Jan;42(1):178–181. doi: 10.1099/00207713-42-1-178. [DOI] [PubMed] [Google Scholar]
- Von Graevenitz A., Osterhout G., Dick J. Grouping of some clinically relevant gram-positive rods by automated fatty acid analysis. Diagnostic implications. APMIS. 1991 Feb;99(2):147–154. doi: 10.1111/j.1699-0463.1991.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Zuber P. L., Gruner E., Altwegg M., von Graevenitz A. Invasive infection with non-toxigenic Corynebacterium diphtheriae among drug users. Lancet. 1992 May 30;339(8805):1359–1359. doi: 10.1016/0140-6736(92)92004-y. [DOI] [PubMed] [Google Scholar]