Abstract
Introduction
Several factor (F) VIII products of different origin and structure are being used for hemophilia A treatment worldwide. The assessment of FVIII concentration in these products is done using activity assays, which are dependent upon the assay and its modifications.
Aim
To evaluate FVIII products for the potency and for FVIII concentration and specific activity.
Methods
Three activity (APTT, intrinsic FXase and synthetic coagulation proteome) and two immunoassays (ELISA and western blotting) were used in this study with albumin-free full-length recombinant (r) FVIII as a standard.
Results
In all activity assays, products A and B (both contain full-length rFVIII) at 1 U/ml showed potency similar to that of the 0.7 nM (1 U/ml) rFVIII standard. Product E (contains truncated rFVIII) was less potent in the APTT (83% of standard) and product C (contains plasma FVIII) was less potent in FXase assays (66%). The ELISA immunoassay revealed that the specific activity of FVIII proteins in products A-C and E varied over a wide range (3,900-13,200 U/mg) and was higher for most lots than that for the standard (5,000 U/mg), whereas the specific activity of product D (contains plasma FVIII) was lower than expected (3,200-4,800 U/mg).
Conclusions
1) FVIII potency estimated in different assays gives dissimilar results; 2) the specific activity of FVIII in various FVIII products is different and inconsistent. Thus, the administration of an equal FVIII potency in units means the administration of different amounts of FVIII protein, which may partly explain apparent discrepancies in product performance.
Keywords: factor VIII activity, factor VIII antigen, factor VIII products, intrinsic factor Xase, thrombin generation, tissue factor
Introduction
The most common genetic bleeding disorder results from deficiency of FVIII coagulant activity (hemophilia A), which occurs in one in 5,000-10,000 male births [1]. In the past, the principal treatments for hemophilia relied on partially purified concentrates of coagulation factors [2,3]. These concentrates, however, have been associated with viral infection transmission [4-6]. During the last two decades, virally inactivated plasma-derived, monoclonal antibody-purified FVIII concentrates that contain negligible amounts of other coagulation factors [7,8], and several rFVIII materials produced by a variety of cell lines with different structures and formulations have been approved and are available for clinical use [9-13].
While the development of the new generations of FVIII products has brought substantial improvements in viral safety, these products have been expensive to develop and over 90% of the cost of hemophilia care is associated with the use of concentrates [14].
Clotting factor concentrates are produced in licensed facilities and each lot of product is assayed for clotting factor activity during the production cycle and designated with a specific potency. This lot potency designation is needed for dosage calculations and is used by the prescribing physician in decisions associated with the prevention and management of prophylaxis and hemorrhagic episodes as well as during and after surgery. Therefore the assay technology employed in the designation of potency must be as accurate as possible and should be standardized thus linking patient care using one concentrate with that of others [15]. Clearly, reliable methods for the meaningful quantitation of FVIII in therapeutic products are essential. Presently, there are two methods used for the evaluation of FVIII potency: clotting and chromogenic assays [16-19]. Substantial discrepancies between FVIII assays have been reported from different pharmacokinetic studies of FVIII concentrates. These discrepancies may in part be caused by differences in standards, reagents, procedures, the recipient and, potentially, by the nature of the FVIII products themselves [17-21]. Moreover, the clotting assay (APTT; “contact pathway” initiated), albeit useful for hemophilia identification, is mechanistically irrelevant to tissue factor (TF)-initiated hemorrhage control and can potentially lead to results different from those observed in in vivo hemostasis. The chromogenic assay, in turn, uses relatively high concentrations of thrombin for FVIII activation at high (nM) preexistent FIXa concentrations. Under physiological conditions, however, factor VIII is activated by extremely low amounts of thrombin produced in situ [22] in the presence of competing substrates and low (pM) concentrations of FIXa.
In this study, we evaluated five pharmacologic FVIII products (3 lots of each) containing both natural and recombinant FVIII proteins in one commercially available (APTT) and in two in-house (FXase and synthetic coagulation proteome [23,24]) activity-based assays. The FVIII protein content in these products was estimated using an in-house immunoassay (ELISA), which allows the quantitation of FVIII antigen in human plasma and in pharmacologic products [25]. The comparison of FVIII products thus entails both potency and specific activity based quantitative analyses.
Materials and Methods
Materials
Human coagulation factors VII, X, IX, and prothrombin were isolated from fresh frozen plasma using the methods of Bajaj et al. [26] and FXI using the monoclonal anti-FXI antibody α-FXI-2. All preparations were purged of trace contaminants and traces of active enzymes as described [24]. Human FV and AT-III were isolated from freshly frozen citrated plasma [27,28]. Citrated plasma for protein purification and von Willebrand factor (vWF) were purchased from Haematologic Technologies, Inc. (Essex Junction, VT). Albumin-free rFVIII and FVIII products A-E were commercially acquired and provided by Baxter Healthcare Corp. (Duarte, CA). Products A and B contain full-length rFVIII, products C and D contain plasma-derived FVIII and product E contains truncated rFVIII. The 7th International Standard FVIII Concentrate (NIBSC code:99/678) was purchased from the National Institute for Biological Standards and Control (Hertfordshire, UK). In all experiments, recombinant full-length albumin-free FVIII produced in Chinese Hamster Ovary cell line was used as a standard. The concentration of this product (0.62 mg/ml) was established by the absorbance at 280 nm using an extinction coefficient E0.1% value of 1.3 [29]. rTF1-242 (expressed in E. coli) was provided as a gift from Drs. Shu Len Liu and Roger Lundblad (Baxter Healthcare Corp). Human rFVIIa was provided as a gift by Dr. Ula Hedner (Novo Nordisk, Denmark). Full-length recombinant tissue factor pathway inhibitor (TFPI) was a gift from Dr. K. Johnson (Chiron Corp, Emeryville, CA). Corn trypsin inhibitor (CTI) was isolated from corn [30]. 1,2-Dioleolyl-sn-Glycero-3-Phospho-L-Serine (PS) and 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (PC) were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA), HEPES and NaCl were purchased from Sigma (St Louis, MO). Phospholipid vesicles (PCPS) composed of 25% PS and 75% PC were prepared as described [31]. TF was relipidated into PCPS vesicles (molar ratio 1:5000) as described [30]. Spectrozyme TH and Spectrozyme FXa were purchased from American Diagnostica, Inc (Greenwich, CT). D-Phe-Pro-ArgCH2Cl (FPRck) and human α-thrombin were produced in house. HRP-sheep anti-mouse Ig was purchased from Amersham Life Sciences (Arlington Heights, IL). Human FIXa and FXa were provided as gifts by Dr. R. Jenny (Haematologic Technologies, Inc., Essex Junction, VT). FVIII/vWF-immunodepleted plasma was purchased from Precision Biologic (Dartmouth, NS, Canada).
Monoclonal anti-FVIII antibodies
Monoclonal anti-FVIII B-domain antibody 1 (αFVIII-1) and biotin-labeled monoclonal anti-FVIII heavy-chain antibody 24 (αFVIII-24) were produced in house and monoclonal anti-FVIII light-chain antibody 68 (αFVIII-68) was produced by Dr. D. Fass. The production and characterization of αFVIII-68 was reported previously [32]. All anti-FVIII clones exhibited apparent affinities for FVIII in the range of 50-160 nM. Epitope mapping using full-length rFVIII, activated rFVIII (rFVIIIa), truncated rFVIII and vWF-containing FVIII product D in conjunction with immunoblotting indicated that αFVIII-1 recognizes an epitope in the amino-terminus of the FVIII B-domain. Based upon the ability of αFVIII-24 to bind rFVIIIa and FVIII in complex with vWF, it appears that its binding region is located in the heavy chain of FVIII. This antibody binds purified full-length rFVIII, rFVIIIa and FVIII in products A-E equally well.
Methods
Immunoassays
A. ELISA
The assay utilized a double-sandwich format using αFVIII-68 as a capture antibody and biotin-labeled αFVIII-24 as a probe [25]. αFVIII-68 was immobilized in the wells of microtiter plate (50 μl/well at 5 μg/ml concentration). Sequential dilutions of FVIII products were added to αFVIII-68-coated wells. FVIII binding was probed with biotin-labeled αFVIII-24 (50 μl/well at 5 μg/ml concentration) and detected using HRP-streptavidin and a chromogenic substrate. The concentration of FVIII protein in the products was estimated from the calibration curve built with the albumin-free recombinant FVIII (standard). The detection limit of this assay is 30 pM FVIII.
B. Western blotting
I. Evaluation of FVIII concentration in FVIII products
2, 1 and 0.5 units of FVIII standard (200, 100 and 50 ng, respectively) and the same units of each FVIII product as indicated by manufacturer were loaded for SDS-PAGE (4-12% gradient) at non-reducing conditions. Western blotting was performed using αFVIII-68 as a primary antibody and HRP-sheep anti-mouse Ig as a secondary. The concentration of FVIII in the products was evaluated by densitometry comparing test bands with those of FVIII standard.
II. Purity of FVIII standard
20 μg of FVIII standard were loaded for SDS-PAGE (4-12% gradient) for the Coomassie Blue staining and 200 ng for western blotting (both at reducing conditions). Western blotting was performed using a mixture of αFVIII-1 and αFVIII-68 as primary antibodies.
APTT
100 μl of substrate plasma (FVIII/vWF-immunodepleted reconstituted with 10 μg/ml purified vWF) containing test sample was incubated for 5 min at 37° with 100 μl of the APTT reagent (Trinity Biotech PLC Bray Co, Wicklow, Ireland). Clotting was initiated by the addition of 100 μl 25 mM CaCl2 and clotting times were determined using the ST8 instrument (Diagnostica Stago, Parsippany, NJ). To build a calibration curve, FVIII-deficient substrate plasma was reconstituted with various concentrations (21-630 pM) of albumin-free full-length rFVIII. For the evaluation of FVIII potency in products, the substrate plasma was reconstituted with 0.03-0.9 U/ml of FVIII product.
Intrinsic FXase
20 μM PCPS, 10 nM FIXa, 1 U/ml FVIII product (0.7 nM for the FVIII standard, i.e. albumin-free full-length rFVIII) and 10 nM thrombin were added to preheated (37 °C) HBS (20 mM HEPES, 150 mM NaCl), 2 mM CaCl2, 0.1% PEG, pH 7.4. FX at 340 nM was added immediately, 20 μl aliquots were removed every 15 s for 4 min and quenched into 160 μl HBS, 20 mM EDTA, 0.1% PEG, pH 7.4. After the completion of the reaction, 20 μl of 2 mM Spectrozyme FXa were added, and the rate of substrate hydrolysis was evaluated. The concentration of FXa at each time-point was estimated from the FXa calibration curve. Maximum rates of FXa generation were calculated for each FVIII product tested. The maximum rate of FXa generation for the albumin-free FVIII standard was used as the reference (100% activity).
Stoichiometry
For the FVIIIa/FIXa stoichiometry measurements, FIXa was used at 1 nM, FX at 340 nM and FVIII at varying concentrations (0-40 nM; established by ELISA). For each FVIII concentration, the maximum rate of FXa generation was evaluated. Apparent functional dissociation constants and binding stoichiometries were calculated using the nonlinear least square regression program ENZFITTER (Biosoft, Cambridge, UK).
Synthetic Coagulation Proteome
The procedure used was a modification of Lawson et al. [23] and van 't Veer et al. [24]. Relipidated TF at 5 pM final concentration was added to the mixture of factors V, VII, VIIa, IX, X, and XI, prothrombin, TFPI and AT-III (all at mean physiologic concentrations) and FVIII at either 1 U/ml (indicated by manufacturer) or 0.7 nM (established by ELISA) in HBS, 2 mM CaCl2 containing 2 μM PCPS. Thrombin generation over time was measured in a chromogenic assay using Spectrozyme TH and a Molecular Devices (Sunnyvale, CA) THERMOmax microplate reader.
Results
Properties and quantitation of the rFVIII “standard”
The molar concentration of the full-length albumin-free rFVIII (rFVIII standard) based upon absorbance at 280 nm was quantitated by functional activity, which provided 1:1 stoichiometry with FIXa determined in the intrinsic FXase assay (Figure 1A). Titration of this rFVIII standard in substrate plasma at concentrations ranging from 21 pM (0.03 U/ml) to 0.63 nM (0.9 U/ml) gave APTT clotting times similar to those observed for corresponding concentrations of the 7th International Standard FVIII Concentrate (Figure 1B). It was also established by gel electrophoresis and western blotting that this rFVIII standard is of a high purity, containing no degradation products beyond the routine heterogeneity of the heavy chain [33] (Figure 1C).
Fig. 1.
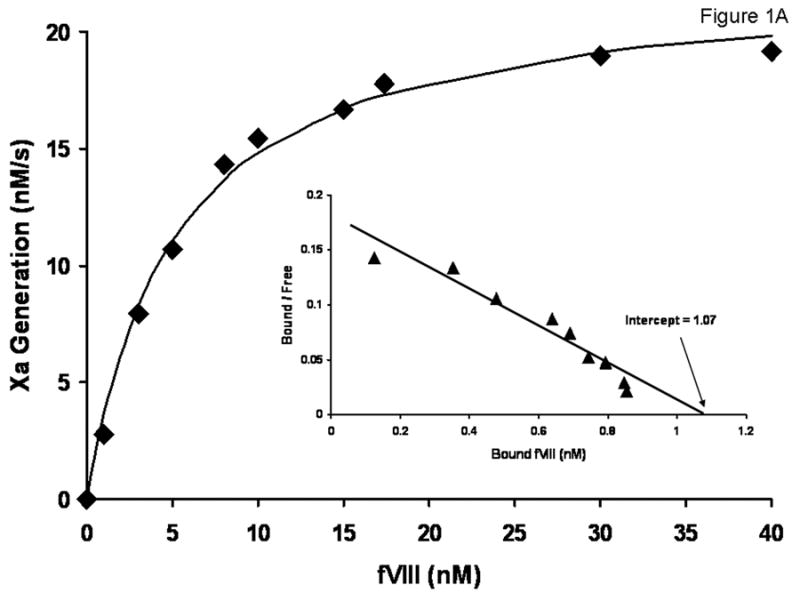
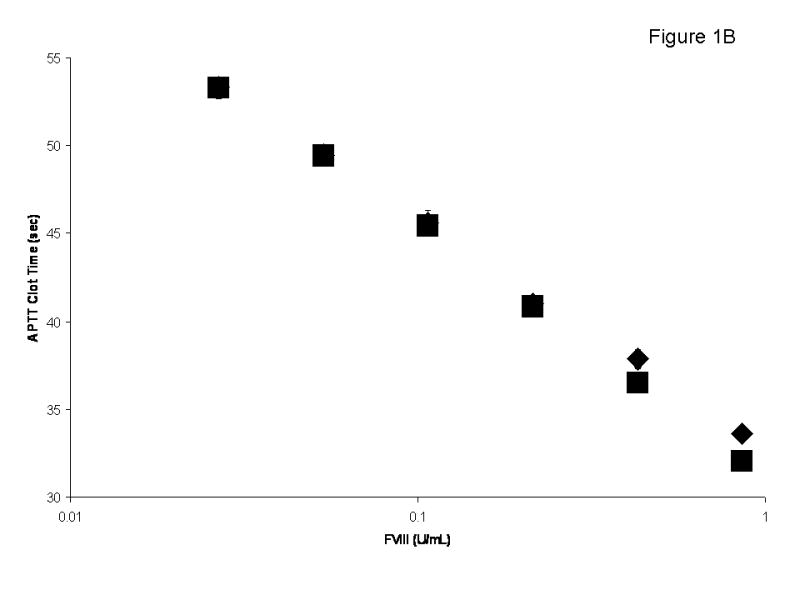
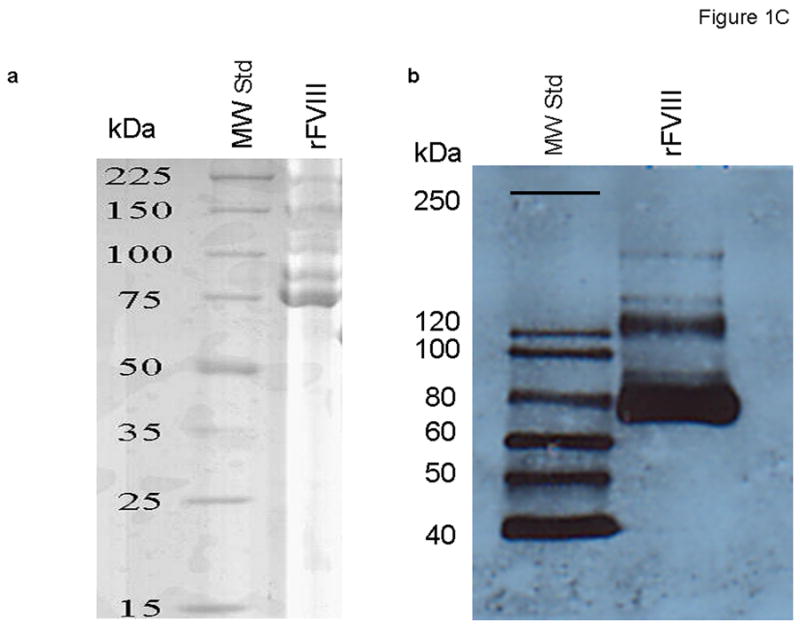
(A) Binding stoichiometry between albumin-free full-length rFVIIIa (FVIII standard) and FIXa. Varying concentrations of FVIII were added to 1 nM FIXa and 20 μM PCPS, followed by the addition of 10 nM thrombin and 340 nM FX. The maximum rate of FXa generation was evaluated for each FVIII concentration and the stoichiometry was calculated using Scatchard transformation. (B) Titration of the 7th International Standard (◆) and albumin-free rFVIII (■) in FVIII-deficient plasma in the APTT assay. (C) SDS-PAGE (a) and western blot (b) of FVIII standard. αFVIII-1 (B domain specific) and αFVIII-68 (light chain specific) were used as primary antibodies for b.
The rFVIII standard was used for the determination of FVIII mass concentration in all FVIII products by an in-house ELISA assay. The data of Figure 2 indicate that immuno-reactivity of all products is equivalent and indistinguishable from that of the rFVIII standard in the range from 50 pM to 1 nM. Based upon the molar concentrations of FVIII in products and their potency identified by the manufacturers and the APTT assay in our laboratory, the specific activity of FVIII in each product was calculated (Table 1).
Fig. 2.
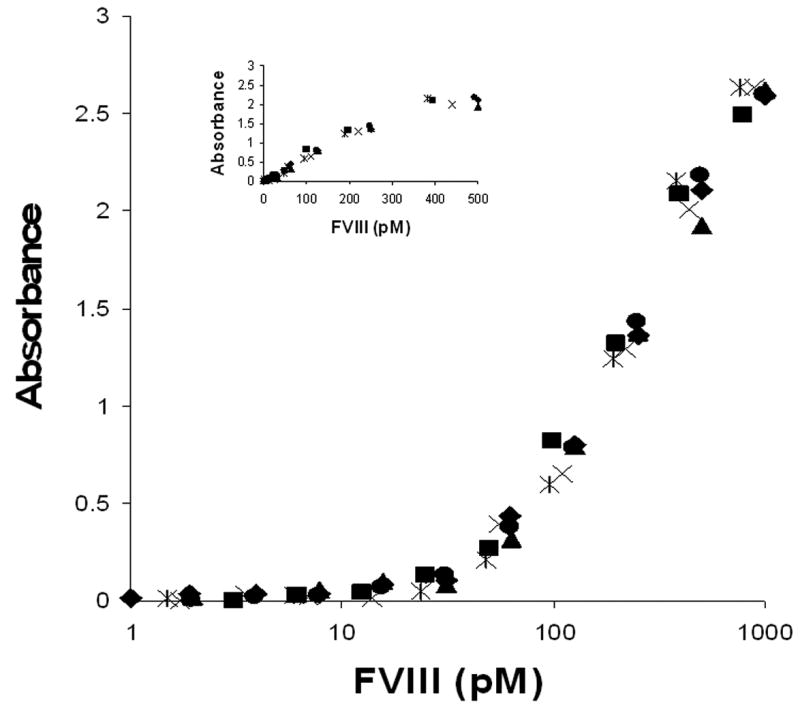
Titration of FVIII standard (◆), product A, lot 2938A540AC (▲), product B, lot GAWSF1 (■), product C, lot 2935B054EA (✖), product D, lot 09H4401K (✱) and product E, lot 77269 (●) in ELISA assay.
Table 1.
Potency and specific activity of FVIII in pharmacologic products
| Product/lot | Potencya U/ml |
FVIII conc.b nM |
APTTc | FXasec | ||
|---|---|---|---|---|---|---|
| Potency
U/ml |
Specific activityd U/mg |
Potency
U/ml |
Specific activityd U/mg |
|||
| rFVIII standard | 3,100 | 2,175 | 3,100 | 5,000 | 3,100 | 5,000 |
| Product A (contains full-length rFVIII) | ||||||
| 2938A540AC | 200 | 88 | 190 | 7,600 | 192 | 7,700 |
| 2938C509AB | 200 | 76 | 190 | 8,700 | 186 | 8,600 |
| 2938C828AA | 200 | 86 | 200 | 8,200 | 200 | 8,200 |
| Average: | 200 | 83 | 193 | 8,200±500 | 193 | 8,200±400 |
| Product B (contains full-length rFVIII) | ||||||
| BXNOL9P | 100 | 30 | 75 | 8,800 | 98 | 11,500 |
| BXNOL7G | 200 | 180 | 200 | 3,900 | 208 | 4,100 |
| GAWSF1 | 400 | 230 | 460 | 7,000 | 388 | 5,900 |
| Average: | 230 | 150 | 245 | 6,600±2,000 | 231 | 7,200±3,200 |
| Product C (contains plasma FVIII) | ||||||
| 2935B054EA | 200 | 105 | 200 | 6,600 | 136 | 4,500 |
| 2935B044EA | 200 | 80 | 170 | 7,500 | 128 | 5,600 |
| 2935Y004EB | 200 | 85 | 160 | 6,600 | 128 | 5,300 |
| Average: | 200 | 90 | 180 | 6,900±400 | 131 | 5,100±500 |
| Product D (contains plasma FVIII) | ||||||
| 09H3901I | 100 | 106 | 140 | 4,600 | 96 | 3,200 |
| 09H3702K | 100 | 100 | 115 | 4,000 | 104 | 3,600 |
| 09H4401K | 100 | 103 | 140 | 4,800 | 96 | 3,300 |
| Average: | 100 | 103 | 132 | 4,500±300 | 99 | 3,400±200 |
| Product E (contains truncated rFVIII) | ||||||
| 77269 | 200 | 95 | 160 | 9,600 | 192 | 11,900 |
| 80431 | 200 | 85 | 170 | 11,400 | 202 | 14,000 |
| 80354 | 200 | 89 | 166 | 10,600 | 200 | 13,200 |
| Average: | 200 | 90 | 165 | 10,500±700 | 198 | 13,000±900 |
Provided by manufacturers;
Determined by ELISA;
Determined in the current study;
Calculated using MW of 285 kDa for rFVIII standard and products A-D and 170 kDa for product E.
Potency of FVIII products
APTT
Titration of the rFVIII standard (Fig. 1B) into congenital FVIII-deficient plasma was used to build the standard curve. A value of 100% potency was assigned to the standard (Figure 3A; Table 1). Pooled plasma (10 donors) showed 115% of potency, equivalent to that of 0.8 nM rFVIII standard. When products were assayed based upon the potency assigned by the manufacturer, the closest in expected potency to the rFVIII standard was product A, which contains full-length rFVIII produced by the same expression system as the standard. The average potency of the three lots was 97±3% of the rFVIII standard with a relatively narrow range between the different lots (95-100%). A higher variability (97±20%; range 75-115%) was observed for product B, another full-length rFVIII product. Product C, which contains FVIII purified from plasma, had an average potency of 88±10% with 80-100% variability. Another plasma FVIII product, product D, had a relatively high potency exceeding at 1 U/ml that of the rFVIII standard by 15-40% (average potency of three lots was 132±14%). The lowest potency in this assay was observed for the truncated rFVIII-containing product E. The average potency of three lots was 83±3%, with a variation range from 80 to 85%.
Fig. 3.
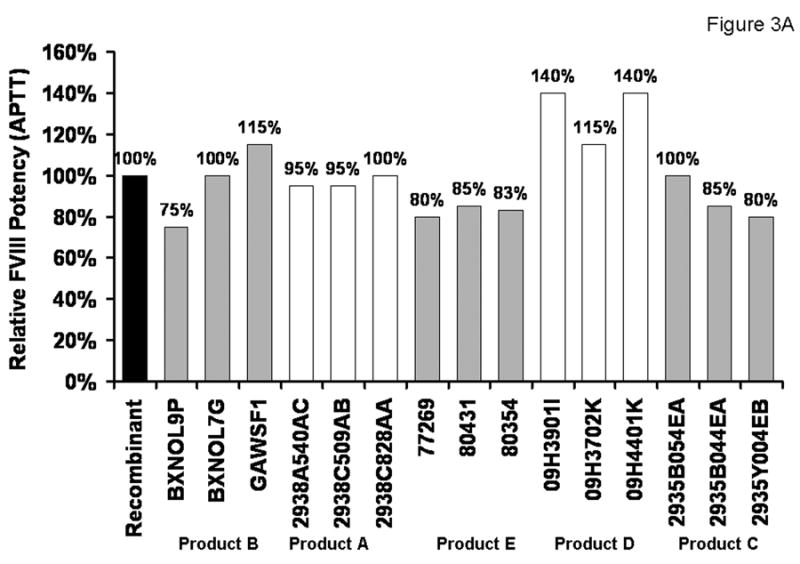
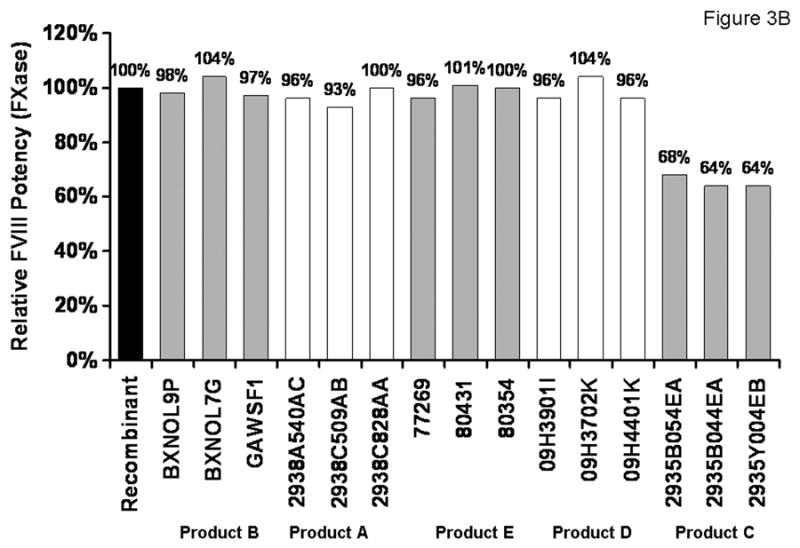
Relative potency of FVIII products in the APTT assay (A) and in the intrinsic FXase (B). 100% was assigned to the potency of 0.7 nM rFVIII standard.
Intrinsic FXase
The rate of FXa generation by the intrinsic FXase in the chromogenic assay (see Methods) containing 0.7 nM (1 U/ml) rFVIII(a) standard was 6.3 nM•s-1. This rate was similar to that published previously [34] and was assigned 100% potency (Figure 3B; Table 1). In this assay, all five FVIII products were evaluated for their relative potency at 1 U/ml (assigned by the manufacturers). Four of the five products tested (products A, B, D and E) had potencies close to that for the rFVIII standard. Inter-product potency variations were relatively small and ranged from 96±4% of rFVIII standard for product A to 100±4% for product B. Similarly, intra-product potency varied in a relatively narrow range (SD<5%) between lots with the highest and the lowest potency. The lots of product C were the least potent in this assay with an average potency of 65±2% for three lots tested.
Synthetic coagulation proteome
After comparing FVIII products in standard assays, we evaluated them in the synthetic coagulation proteome, which attempts to replicate the protein chemistry of the extrinsic pathway, and allows the measurement of thrombin generation during the entire course of the reaction; in contrast to the APTT assay, which stops at the inception of the propagation phase of the thrombin generation process with ≤2% prothrombin converted to thrombin [35,36]. Thrombin generation in the synthetic coagulation proteome was evaluated either with 1 U/ml of FVIII product or with 0.7 nM FVIII (based on the immunoassay) present in each product. In both cases, the rFVIII standard at 0.7 nM concentration was used for the comparison of FVIII potency in the products.
Robust thrombin generation triggered with 5 pM relipidated TF in the reaction mixture with 0.7 nM rFVIII standard started after 5 min of the initiation phase (Figure 4A). The maximum level of thrombin observed was 405 nM. In the absence of FVIII, thrombin generation during the propagation phase and the maximum concentration of thrombin observed in the reaction (<100 nM) was significantly suppressed. The substitution of the 0.7 nM rFVIII standard for any lot of product A at 1 U/ml did not significantly alter the thrombin generation profile. Little variation in thrombin generation was observed for three lots of product A. Similar results were obtained for products D (Fig. 4B), B, C, and E at 1 U/ml (potency provided by the manufacturer; data not shown).
Fig. 4.
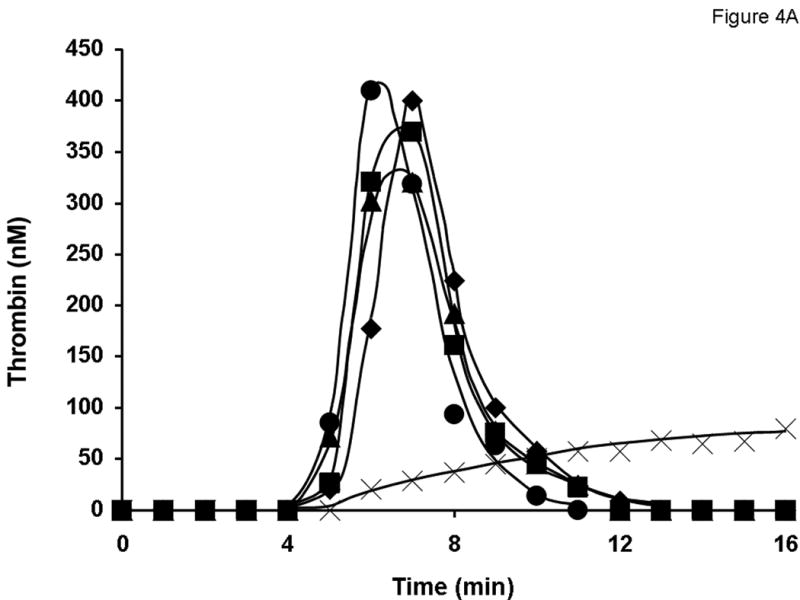
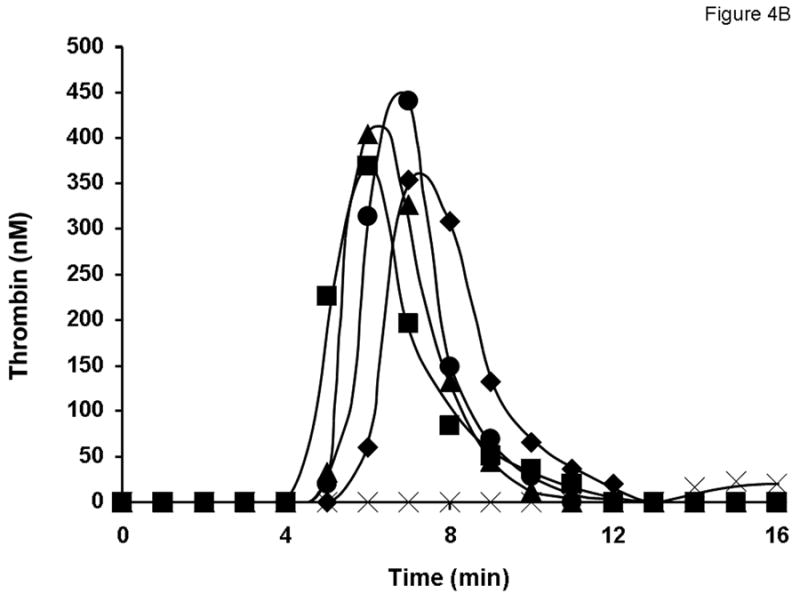
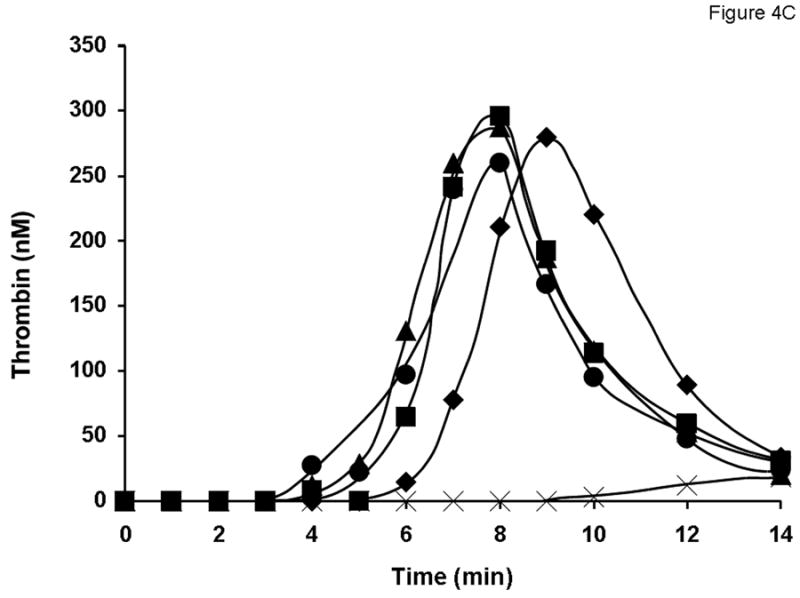
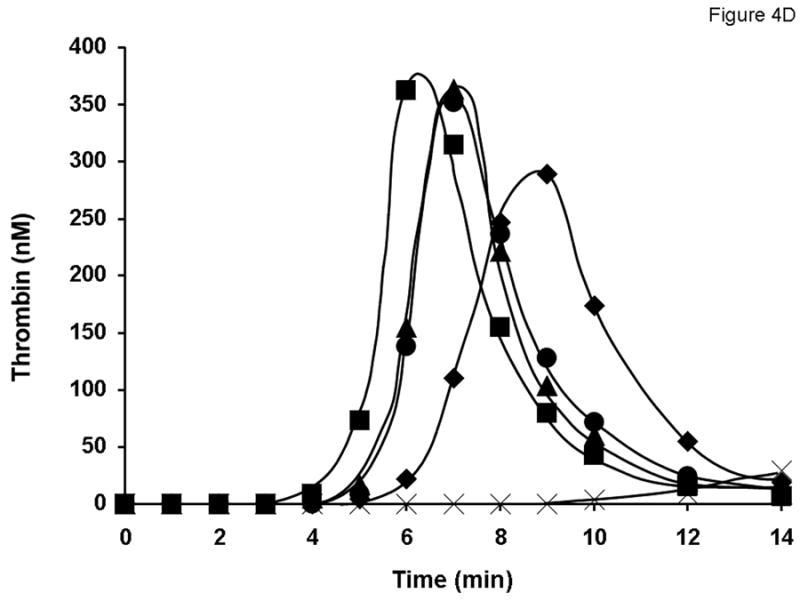
Evaluation of different lots of product A (A and C) and product D (B and D) in the synthetic coagulation proteome with 2 μM PCPS. Thrombin generation was triggered with 5 pM relipidated TF in the presence of 0.7 nM FVIII standard (◆) or product A lot 2938A540AC (■), lot 2938C509AB (●) and lot 2938C828AA (▲) either at 1 U/ml (A) or at 0.7 nM FVIII (C) and product D lot 09H3901I (■), lot 09H3702K (●) and lot 09H4401I (▲) at 1 U/ml (B) or 0.7 nM (D). ✕ represents thrombin generation in the absence of FVIII.
Concentration and specific activity of FVIII protein in FVIII products
To evaluate the specific activity of FVIII in various products, the products were evaluated for the molar concentration (mass) of FVIII protein using the immunoassays. Both ELISA (Table 1) and western blotting (not shown) gave similar results. For products D, E and A, the inter-lot concentration of FVIII protein varied in a relatively narrow range (SD 2, 4 and 6%, respectively). For product C, the variability increased to 12% and for product B FVIII concentration (per unit of potency) varied in a wide range (SD 40%).
The differences in specific activities for FVIII products in general as well as for individual lots closely reflect the differences observed for FVIII mass in those products (Table 1). Four of the five products showed a relatively low inter-lot variability for the FVIII specific activity in those products. The exception was product B, which showed a pronounced inter-lot variability for specific activity determined in both the APTT (6,600±2,000 U/mg) and FXase (7,200±3,200 U/mg) assays. The inter-product specific activity of FVIII varied in a wide range, from 3,400±200 U/mg for product D to 13,000±900 U/mg for product E. A comparison of two methods (APTT and FXase) used for the determination of specific activity of FVIII did not show any particular trend. For example, for product A, the specific activity of FVIII for all three lots was similar in both assays. For products C and D, a significantly higher specific activity was determined in the clotting assay, whereas for product E this parameter showed a higher value in the FXase.
All 15 lots of FVIII products were tested at an equimolar FVIII protein concentration (0.7 nM; established by ELISA) in the synthetic coagulation proteome. Consistent with their higher specific activity, all lots of products A (Fig. 4C), C and E (not shown) lead to a faster and more robust thrombin generation than that observed for 0.7 nM of the rFVIII standard. For product B (not shown) at 0.7 nM, thrombin generation profiles for all three lots were similar to that observed for the rFVIII standard. Interestingly, all three lots of product D, which had specific activities lower than that of the rFVIII standard, were more active than the latter with respect to both the initiation phase duration and thrombin generation rate (Fig. 4D).
Discussion
The data of this study indicate that using a well-characterized (purity, concentration and activity) full-length albumin-free rFVIII as a standard, only one of five FVIII products tested (product A) had a potency specified by the manufacturer consistent with the product's performance in three activity-based assays. This is not surprising because the FVIII present in product A and rFVIII standard is the same protein formulated to meet the requirements of a pharmacologic product. The other four FVIII products displayed pronounced inter-assay variability. Product E showed a higher potency in the intrinsic factor Xase than in the clotting assay, whereas the two plasma FVIII products (C and D) displayed a higher potency in the clotting assay. In addition to the inter-assay potency variation, product B demonstrated a pronounced variation of inter-lot potency in the APTT.
It is easy to notice that using FVIII products at 1 U/ml in the FXase assay, the observed potency was similar to that of the rFVIII standard for four of five products tested with a relatively low inter-lot variability for all five products (see Figure 3B and Table 1). In contrast, the inter-product variability and the inter-lot variability for three of five products were more pronounced when the APTT assay was used for the evaluation of their potency (compare Figures 3A and 3B), suggesting that the FXase assay is more reproducible than the APTT.
One potential important difference between these two assays is related to the formation and stability of the intrinsic FXase complex. At clotting time in the APTT assay, the concentration of FIXa generated is extremely low (2-5 pM) [23,37], which is far from saturating. Additionally, although FVIII is rapidly activated by thrombin, a spontaneous inactivation of FVIIIa caused by the dissociation of the A2 subunit is also a rapid process [38]. As a consequence, the concentration of the FVIIIa-FIXa complex never reaches a steady value during the processes leading to plasma clotting and is sensitive to the composition of the test plasma as well to any (even minor) variations in the assay procedure.
In the intrinsic FXase assay, a relatively high concentration of FIXa is used (10 nM) and due to a high affinity (70 pM) between FVIIIa and factor IXa in the presence of phospholipids [39], all FVIIIa formed is incorporated into the intrinsic Xase. At this FIXa concentration FVIIIa remains stable throughout the entire assay [40]. As a result, the activity of the intrinsic FXase becomes solely dependent upon the concentration and specific activity of FVIII used in the assay.
Analyses of the FVIII antigen concentration by immunoassays [25] revealed that there is little (if any) correlation between the potency of the product and FVIII mass concentration. This observation is similar to that made by Lin and coworkers [41]. In the current study, we observed that the molar FVIII protein concentrations in various products, which were identified by the manufacturer as containing 1U/ml potency, varied from 0.38 nM for one lot of product A to 1.1 nM for one lot of product D. In general, all products except product D had higher FVIII specific activities than that observed for the rFVIII standard (5000 U/mg). The inter-lot specific activity for four of five products varied over a relatively narrow range, whereas the specific activity of product B showed almost 3-fold difference between the lots with the highest and the lowest values of this parameter.
In conclusion, the administration of equal FVIII potency based upon the manufacturer's assessment involves the administration of different amounts of FVIII protein. It is not clear how this observation may impact the efficient treatment of hemophilia A patients. The data of this study suggest somewhat paradoxically that products with higher FVIII protein content per unit of activity may, possibly, be more efficient than those, which contain FVIII of a higher specific activity.
Acknowledgments
This study was supported by P01 HL46703 grant from the National Institutes of Health and by Baxter Healthcare-Bioscience. We thank Dr. U. Hedner (Novo Nordisk), Dr. K. Johnson (Chiron) and Dr. R. Lundblad (Hyland Division, Baxter Healthcare) for providing us with recombinant proteins (FVIIa, TFPI, albumin-free FVIII and TF1-242). All FVIII products were commercially acquired and provided by Baxter Healthcare-Bioscience. We also thank Dr. T. Orfeo for his help with numerical simulations and J. Amblo, M. F. Whelihan and R. Cooley for their technical assistance. This work was in part presented at the XXth Congress of the International Society on Thrombosis and Haemostasis , August 6-12, 2005, Sydney, Australia (abstract number: P2028).
References
- 1.Gitschier J, Kogan S, Diamond C, Levinson B. Genetic basis of hemophilia A. Thromb Haemost. 1991;66:37–9. [PubMed] [Google Scholar]
- 2.Kekwick RA, Wolf P. A concentrate of human antihaemophilic factor - its use in six cases of haemophilia. Lancet. 1957;i:647. doi: 10.1016/s0140-6736(57)91116-9. [DOI] [PubMed] [Google Scholar]
- 3.Hoag MS, Johnson FF, Robinson JA, Aggeler PM. Treatment of hemophilia B with a new clotting-factor concentrate. N Engl J Med. 1969;28:581–6. doi: 10.1056/NEJM196903132801103. [DOI] [PubMed] [Google Scholar]
- 4.Schulman S, Wiechel B. Hepatitis, epidemiology and liver function in hemophiliacs in Sweden. Acta Med Scand. 1984;215:249–56. doi: 10.1111/j.0954-6820.1984.tb05002.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson AR. Factor IX concentrates for clinical use. Semin Thromb Hemost. 1993;19:25–36. doi: 10.1055/s-2007-994003. [DOI] [PubMed] [Google Scholar]
- 6.Chorba TL, Holman RC, Clarke MJ, Evatt BL. Effects of HIV infection on age and cause of death for persons with hemophilia A in the United States. Am J Hematol. 2001;66:229–40. doi: 10.1002/ajh.1050. [DOI] [PubMed] [Google Scholar]
- 7.Fulcher CA, Zimmerman TS. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci USA. 1982;79:1648–52. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croissant MP, van de Pol H, Lee HH, Allain JP. Characterization of four monoclonal antibodies to factor VIII coagulant protein and their use in immunopurification of factor VIII. Thromb Haemost. 1986;56:271–6. [PubMed] [Google Scholar]
- 9.Schwartz RS, Abildgaard CF, Aledort LM, Arkin S, Bloom AL, Brackmann HH, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. Recombinant Factor VIII Study Group. N Engl J Med. 1990;323:1800–5. doi: 10.1056/NEJM199012273232604. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg H, Almstedt A, Brandt J, Gray E, Holmquist L, Oswaldsson U, et al. Structural and functional characteristics of the B-domain-deleted recombinant factor VIII protein, r-VIII SQ. Thromb Haemost. 2001;85:93–100. [PubMed] [Google Scholar]
- 11.Gale AJ, Radtke KP, Cunningham MA, Chamberlain D, Pellequer JL, Griffin JH. Intrinsic stability and functional properties of disulfide bond-stabilized coagulation factor VIIIa variants. J Thromb Haemost. 2006;4:1315–22. doi: 10.1111/j.1538-7836.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 12.Lusher JM. First and second generation recombinant factor VIII concentrates in previously untreated patients: recovery, safety, efficacy, and inhibitor development. Semin Thromb Haemost. 2002;28:273–6. doi: 10.1055/s-2002-32662. [DOI] [PubMed] [Google Scholar]
- 13.Suiter TM. First and next generation native rFVIII in the treatment of hemophilia A. What has been achieved? Can patients be switched safely? Semin Thromb Haemost. 2002;28:278–83. doi: 10.1055/s-2002-32663. [DOI] [PubMed] [Google Scholar]
- 14.Miners AH. The economics of hemophilia treatments. In: Lee CH, Berntorp EE, Hoots KW, editors. Textbook of Hermophilia. Malden MA: Blackwell Publishing; 2005. pp. 351–8. [Google Scholar]
- 15.Barrowcliffe TW. Products used to treat hemophilia. In: Lee CH, Berntorp EE, Hoots KW, editors. Textbook of Hermophilia. Malden MA: Blackwell Publishing; 2005. pp. 242–8. [Google Scholar]
- 16.Kleinveld HA, Andersson NE, van Voorthuiozen H, den Hartog J, de Groot PG. Determination of coagulation factor VIII activity by a chromogenic substrate method on STA, an automated coagulation analyzer. Scand J Clin Invest. 1999;59:335–41. doi: 10.1080/00365519950185526. [DOI] [PubMed] [Google Scholar]
- 17.Mikaelsson M, Oswaldsson U, Jankowski MA. Measurement of factor VIII activity of B-domain deleted recombinant factor VIII. Semin Hematol. 2001;38(2 Suppl 4):13–23. doi: 10.1016/s0037-1963(01)90104-0. [DOI] [PubMed] [Google Scholar]
- 18.Barrowcliffe TW, Raut S, Sands D, Hubbard AR. Coagulation and chromogenic assays of factor VIII activity: general aspects, standardization, and recommendations. Semin Thromb Haemost. 2002;28:247–55. doi: 10.1055/s-2002-32658. [DOI] [PubMed] [Google Scholar]
- 19.Mikaelsson M, Oswaldsson U. Assaying the circulating factor VIII activity in hemophilia A patients treated with recombinant factor VIII products. Semin Thromb Haemost. 2002;28:257–64. doi: 10.1055/s-2002-32659. [DOI] [PubMed] [Google Scholar]
- 20.Lusher JM, Hillman-Wiseman C, Hurst D. In vivo recovery with products of very high purity – assay discrepancies. Haemophilia. 1998;4:641–5. doi: 10.1046/j.1365-2516.1998.440641.x. [DOI] [PubMed] [Google Scholar]
- 21.Mikaelsson M, Oswaldsson U, Sandberg H. Influence of phospholipids on the assessment of factor VIII activity. Haemophilia. 1998;4:646–50. doi: 10.1046/j.1365-2516.1998.440646.x. [DOI] [PubMed] [Google Scholar]
- 22.Butenas S, van 't Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–33. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 23.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269:23357–66. [PubMed] [Google Scholar]
- 24.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem. 1997;272:4367–77. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 25.Parhami-Seren B, Butenas S, Amblo J, Fass DN, Mann KG. Quantitation of FVIII antigen in human plasma. J Thromb Haemost. 2005;3(Suppl 1):P0650. [Google Scholar]
- 26.Bajaj SP, Rapaport SI, Prodanos C. A simplified procedure for purification of human prothrombin, factor IX and factor X. Prep Biochem. 1981;11:397–412. doi: 10.1080/00327488108065531. [DOI] [PubMed] [Google Scholar]
- 27.Katzmann JA, Nesheim ME, Hibbard LS, Mann KG. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci USA. 1981;78:162–6. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith MJ, Noyes CM, Church FC. Reactive site peptide structural similarity between heparin cofactor II and antithrombin III. J Biol Chem. 1985;260:2218–25. [PubMed] [Google Scholar]
- 29.Kumar H, Healey JF, Lollar P, Durrani MJ. Determination of free sulfhydryls in human factor VIII. J Thromb Haemost. 2003;1(Suppl 1):P1067. [Google Scholar]
- 30.Cawthern KM, van 't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–92. [PubMed] [Google Scholar]
- 31.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258:6503–8. [PubMed] [Google Scholar]
- 32.Precup JW, Kline BC, Fass DN. A monoclonal antibody to factor VIII inhibits von Willebrand factor binding and thrombin cleavage. Blood. 1991;77:1929–36. [PubMed] [Google Scholar]
- 33.Fay PJ, Anderson MT, Chavin SI, Marder VJ. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986;871:268–78. doi: 10.1016/0167-4838(86)90208-6. [DOI] [PubMed] [Google Scholar]
- 34.van Dieijen G, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256:3433–42. [PubMed] [Google Scholar]
- 35.Rand MD, Lock JB, van 't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45. [PubMed] [Google Scholar]
- 36.Butenas S, van 't Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–33. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 37.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–33. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 38.Lu D, Kalafatis M, Mann KG, Long GL. Comparison of activated protein C/protein S-mediated inactivation of human factor VIII and factor V. Blood. 1996;87:4708–17. [PubMed] [Google Scholar]
- 39.Mathur A, Zhohg D, Sabharwal AK, Smith KJ, Bajaj SP. Interaction of factor IXa with factor VIIIa. Effects of protease domain Ca2+ binding site, proteolysis in the autolysis loop, phospholipid, and factor X. J Biol Chem. 1997;272:23418–26. doi: 10.1074/jbc.272.37.23418. [DOI] [PubMed] [Google Scholar]
- 40.Lollar P, Knutson GJ, Fass DN. Stabilization of thrombin-activated porcine factor VIII:C by factor IXa and phospholipid. Blood. 1984;63:1303–8. [PubMed] [Google Scholar]
- 41.Lin Y, Yang X, Chevrier MC, Craven S, Barrowcliffe TW, Lemieux R, Ofosu FA. Relationships between factor VIII:Ag and factor VIII in recombinant and plasma-derived factor VIII concentrates. Haemophilia. 2004;10:459–69. doi: 10.1111/j.1365-2516.2004.00957.x. [DOI] [PubMed] [Google Scholar]


