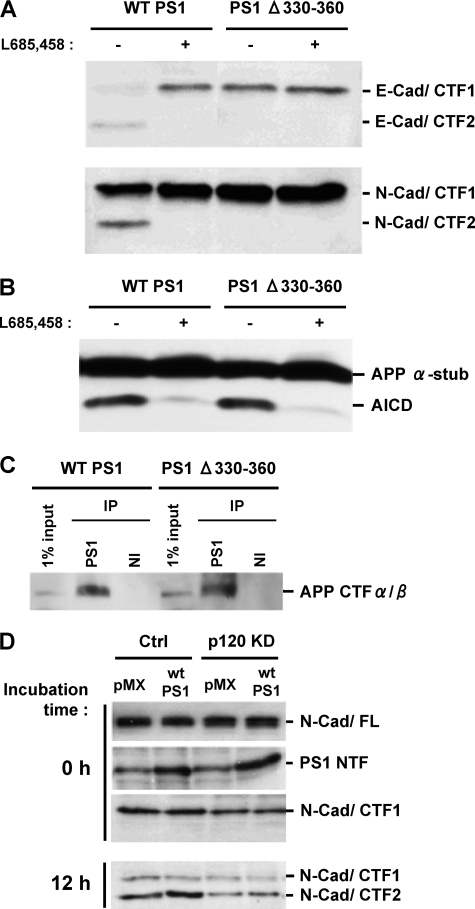
FIGURE 3.
PS1 mutant unable to bind p120ctn fails to promote cadherin processing although it promotes APP processing. A, HEK293 cells transduced with WT PS1 or PS1Δ330–360 were grown to confluency, and membrane fractions were prepared and used to perform the in vitro γ-secretase assay as described (2, 9) at 37 °C for 2 h in the presence (+) or absence (–) of 1 μm γ-secretase inhibitor L665,458. The resulting samples were submitted to SDS-PAGE and probed on WBs with either anti-E- or anti-N-cadherin antibodies to detect the cadherin products indicated at the right of the figure. PS1Δ330–360 defective in p120ctn binding is unable to promote the production of the γ-secretase cleavage products E-cad/CTF2 and N-cad/CTF2. B, in vitro assay for the detection of AICD was performed as described for A using anti-APP/CTF antibody R1 APP α-stub, (APP CTFα/β). C, HEK293 cells transduced with WT PS1 or PS1Δ330–360 were grown to confluency, extracted in Nonidet P-40, and then immunoprecipitated with an anti-PS1 antibody (S182) or nonimmune serum (NI). Obtained samples were submitted to SDS-PAGE, and the immunoblots were probed with anti-APP antibody R1. PS1Δ330–360 binds APP/CTF as efficiently as WT PS1. D, knockdown of p120ctn decreases the γ-secretase processing of cadherins. Control and p120ctn knockdown HEK293 cell cultures were transduced with either vector (pMX) or PS1 (WT PS1) expression constructs. N-cadherin levels in all cultures were adjusted as described in the legend Fig. 2. Membrane fractions from each culture were used for the in vitro γ-secretase assay at 37 °C for 12 h as described for A. The resulting samples were submitted to SDS-PAGE and immunoblotted with anti-N-cadherin or anti-PS1/NTF antibodies to detect the products indicated at the right of the figure. Knockdown of p120ctn reduced production of the γ-secretase product N-cad/CTF2 even in the presence of increased PS1 levels.