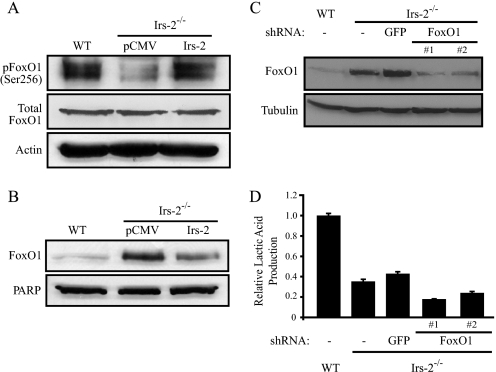
FIGURE 3.
Involvement of FoxO1 in the Irs-2-dependent regulation of aerobic glycolysis. A, aliquots of cell extracts that contained equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with antibodies that recognize phosphoSer256 of FoxO1, total FoxO1, or actin. pCMV, Irs-2–/– cells transfected with vector; Irs-2, Irs-2–/– cells transfected with Irs-2. B, aliquots of nuclear extracts containing equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with a FoxO1-specific antibody. Poly(ADP-ribose) polymerase-2 (PARP) was used as a nuclear loading control. C, FoxO1 expression was suppressed in the PyV-MT:Irs-2–/– cells by lentiviral infection with a FoxO1-specific shRNA. Total cell extracts from WT and Irs-2–/– parental cell lines and the Irs-2–/– cells expressing either a GFP-shRNA or a FoxO1-shRNA (two independent subclones) were immunoblotted with either FoxO1-specific or tubulin-specific antibodies. D, cells were assayed for lactic acid production, and the data are expressed as the ratio of the rate of lactic acid production (mm/mg of total protein/h) relative to WT cells. The data shown represent the mean (± S.E.) of a representative experiment performed in triplicate.