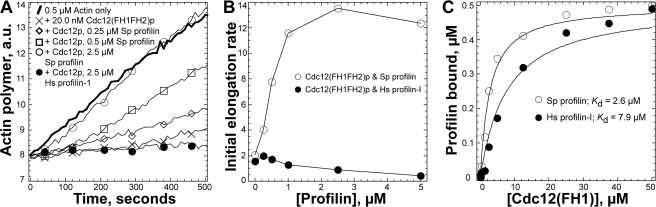
FIGURE 2.
Fission yeast profilin (S. pombe PRF), but not human profilin (HPRF), allows Cdc12p-associated actin filaments to elongate their barbed ends. A, effects of profilins and formin Cdc12(FH1FH2)p on the time course of elongation of the barbed ends of preassembled filament seeds. Conditions: 10 mm imidazole, pH 7.0, 50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 0.5 mm DTT, 0.2 mm ATP, 90 μm CaCl2. Reactions were started by mixing 0.5 μm magnesium-ATP actin monomers (10% pyrene labeled) with 0.5 μm actin filaments: thick curve, actin alone; other samples contained 20 nm Cdc12(FH1FH2)p with: ×, no profilin; ⋄, 0.25 μm S. pombe profilin; □, 0.50 μm S. pombe profilin; ○, 2.5 μm S. pombe profilin; •, 2.5 μm Hs profilin-I. B, dependence of the initial rate of barbed end assembly (slope) with 20 nm Cdc12(FH1FH2)p on the concentrations of (○) S. pombe profilin or (•) Hs profilin-I. C, affinity of profilin for the Cdc12p proline-rich FH1 domain. Conditions: 20 mm Tris, pH 7.5, 150 mm KCl, 0.2 mm DTT. Either 0.5 μm S. pombe profilin (○) or Hs profilin (•) were incubated with a range of concentrations of Cdc12(FH1)p. The intrinsic tryptophan fluorescence of profilin was measured and plotted versus the concentration of Cdc12(FH1)p. Curve fits revealed the indicated equilibrium dissociation constants.