FIGURE 1.
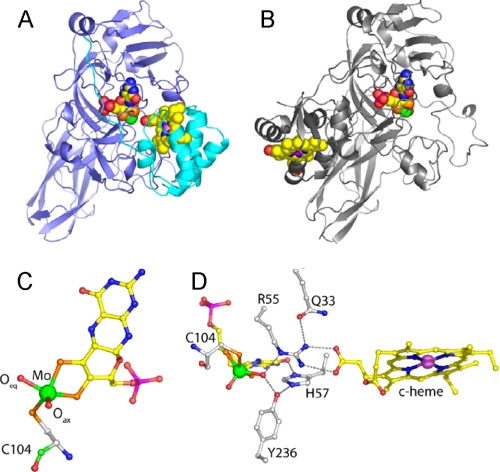
Details of the crystal structure of wild type SDH and comparison with CSO. A, ribbon diagram of the SDH heterodimer with the SorA and SorB subunits colored blue and cyan, respectively, and the redox cofactors in space-filling mode with the molybdenum atom colored green and the iron atom colored violet. B, ribbon diagram of a single subunit of CSO with the molybdopterin binding domain in the same orientation as SorA in A. The cytochrome domain of CSO is clearly in a different position with respect to the molybdenum cofactor than is seen for the cytochrome subunit of SDH. C, SDH molybdopterin cofactor demonstrating the geometry of the molybdenum ligands. The thiol ligands donated by the organic component of molybdopterin and the Cys-104 side chain, and the reactive oxygen ligand (Oeq) sit in the equatorial plane with the axial oxygen (Oax) ligand at the apex of a square pyramid. Atoms are colored as follows: molybdenum (green), sulfur (orange), phosphorous (magenta), oxygen (red), nitrogen (blue), and carbon (yellow in the cofactor and white in the protein). D, hydrogen bonding network around the substrate binding site. The molybdopterin and heme cofactors are shown together with active site residues Cys-104, Arg-55, His-57, Tyr-236, and Gln-33. Figs. 1 and 4 were prepared using Pymol (37).