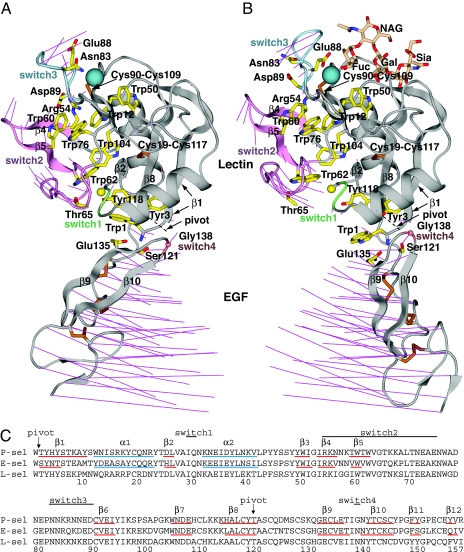
Fig. 2.
Overall conformational movements in selectin allostery. (A and B). Ribbon cartoons of bent conformation (PDB ID code 1G1Q, chain A) (A) and extended conformation (PDB ID code 1G1S, chain A) (B) (7) in identical orientations after superposition on the lectin domain. The sialyl LewisX moiety of PSGL-1 is shown as sticks in wheat. Thin magenta lines in A and B connect the same Cα atoms in the bent and extended structures for all residues. Switch region ribbons are color-coded. Side chains are shown as yellow sticks with blue nitrogens and red oxygens, except cysteine side chains are gold. The Cβ of Ala-28 and Cα of Gly-138 are shown as spheres. The Ca2+ (or equivalent Sr2+) ion is shown as a large teal sphere. (C) Sequence alignment. Secondary structure elements determined with DSSP (24) are marked.