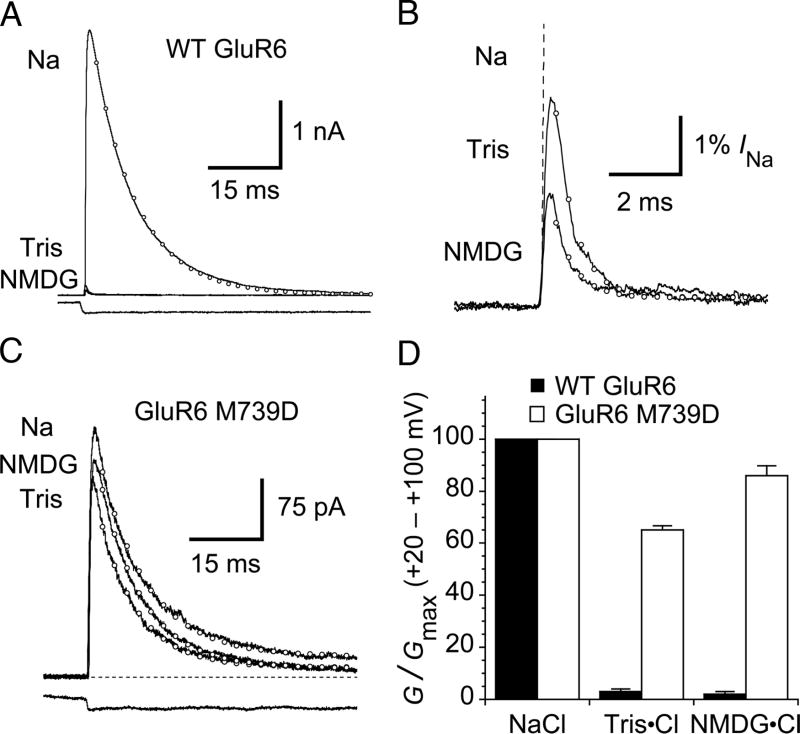
Figure 1. GluR6 M739D mutant is relatively insensitive to NMDG and Tris.
(A) In normal saline (Na), outward currents at + 60 mV due to wild-type GluR6 receptors activate rapidly in response to a 100 ms pulse of 10 mM glutamate and then desensitize (open circles represent a monoexponential fit, kdes = 110 s−1). In the same patch, when external sodium is replaced by either Tris or N-methyl-D-glucamine (NMDG), outward currents are strongly inhibited. The lower trace shows the solution exchange into glutamate, measured after the experiment.
(B) Expanded view of the current response in NMDG and Tris at +60 mV. The response in Na is shown as a dashed line. Monoexponential fits are shown with open circles. The rate of desensitization is ~ 15-fold faster (in Tris, 1600 ± 400 s−1 and in NMDG, 1600 ± 100 s−1 n = 5) than in sodium-containing solutions.
(C) The desensitization and peak current of the GluR6 M739D mutant is much less sensitive to substitution of sodium with NMDG or Tris. Monoexponential fits are shown as open circles (in this patch, Na kdes = 99 s−1; NMDG kdes = 110 s−1; Tris kdes = 124 s−1). The lower trace is the solution exchange measured after the experiment.
(D) Bar plot indicating inhibition of the slope conductance at positive potentials for wild-type and M739D receptors. The limited inhibition of the M739D mutant by NMDG and Tris probably derives in part from channel block. Data are mean ± SEM for five patches.